Rashnonejad Lab
The Rashnonejad Lab focuses on developing adeno-associated virus (AAV)-based gene therapies for neuromuscular and neurodegenerative diseases. Its team works on monogenetic disorders including ACTA1-related Nemaline Myopathy (NEM3), Charcot-Marie-Tooth type 1B (CMT1B), Dejerine–Sottas syndrome (DSS, another form of early onset CMTs), Smith-Kingsmore syndrome (SKS) and mitochondrial disorders. The Rashnonejad Lab is also interested in developing in-utero (prenatal or fetal) gene therapies for severe, congenital neuromuscular disorders. The lab uses cutting-edge technologies such as gene replacement, gene silencing (RNAi), genome editing (CRISPR), and Exon skipping and facilitates their translation into human testing by optimizing AAV vector delivery to target tissues. The team’s research requires the use of cellular and rodent models to investigate disease pathogenesis, vector safety and efficacy, and the identification of biomarkers as therapeutic outcome measures toward translating these therapies to the clinic.
Current Projects
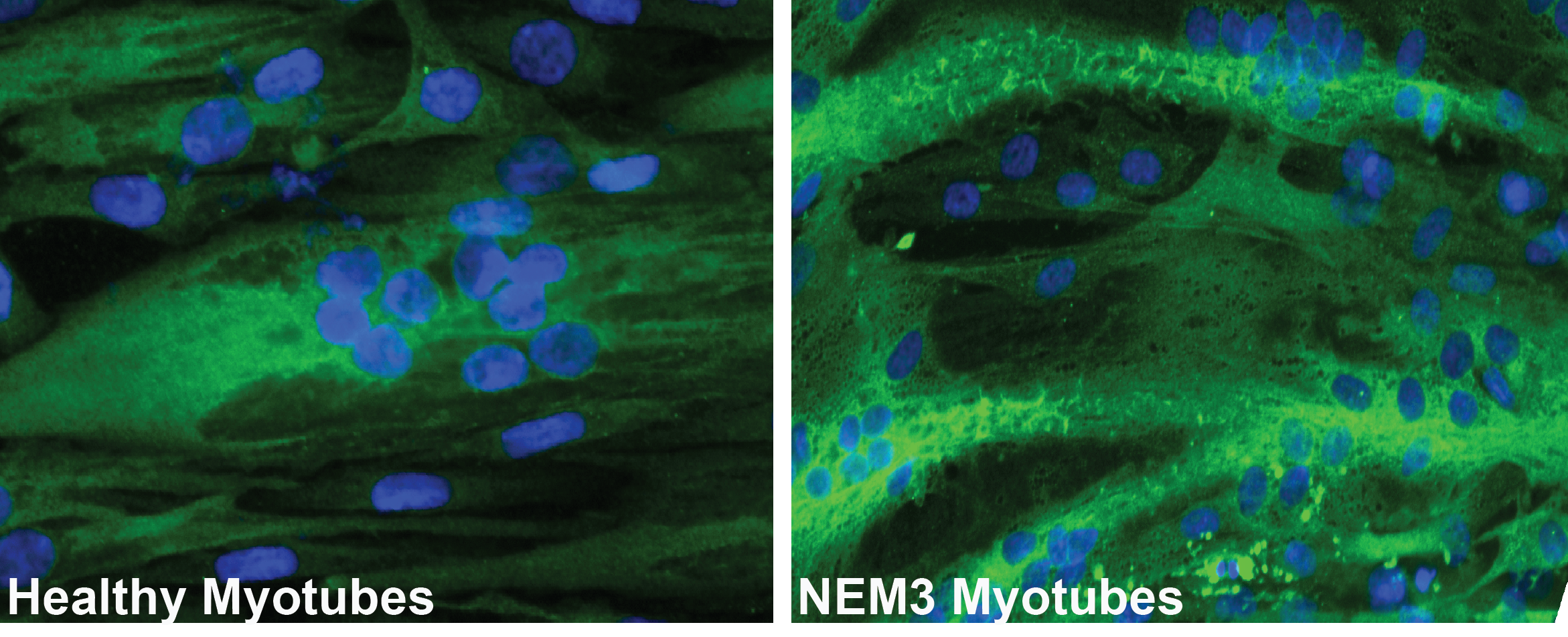
In this figure, we show the accumulation of mutant ACTA1 in human myotubes differentiated from the myoblasts of an ACTA1 patient compared to healthy myotubes.
ACTA1-related Nemaline Myopathy, also known as Nemaline Myopathy type 3 (NEM3), is a congenital myopathy that affects children immediately after birth. Unfortunately, most patients lose their lives before the age of one year. Numerous mutations in the ACTA1 gene have been associated with NEM3. The ACTA1 gene encodes skeletal muscle α-actin, the predominant actin isoform in the sarcomeric thin filaments of adult skeletal muscle, which, along with myosin, is essential for muscle contraction. Mutations in ACTA1 lead to a severe type of congenital myopathy. My group is developing a novel, inducible mouse model for ACTA1 disease, allowing us to study disease mechanisms in various severities in depth. Additionally, we are developing AAV-based gene therapies for NEM3 and testing them on both in vitro and in vivo models.
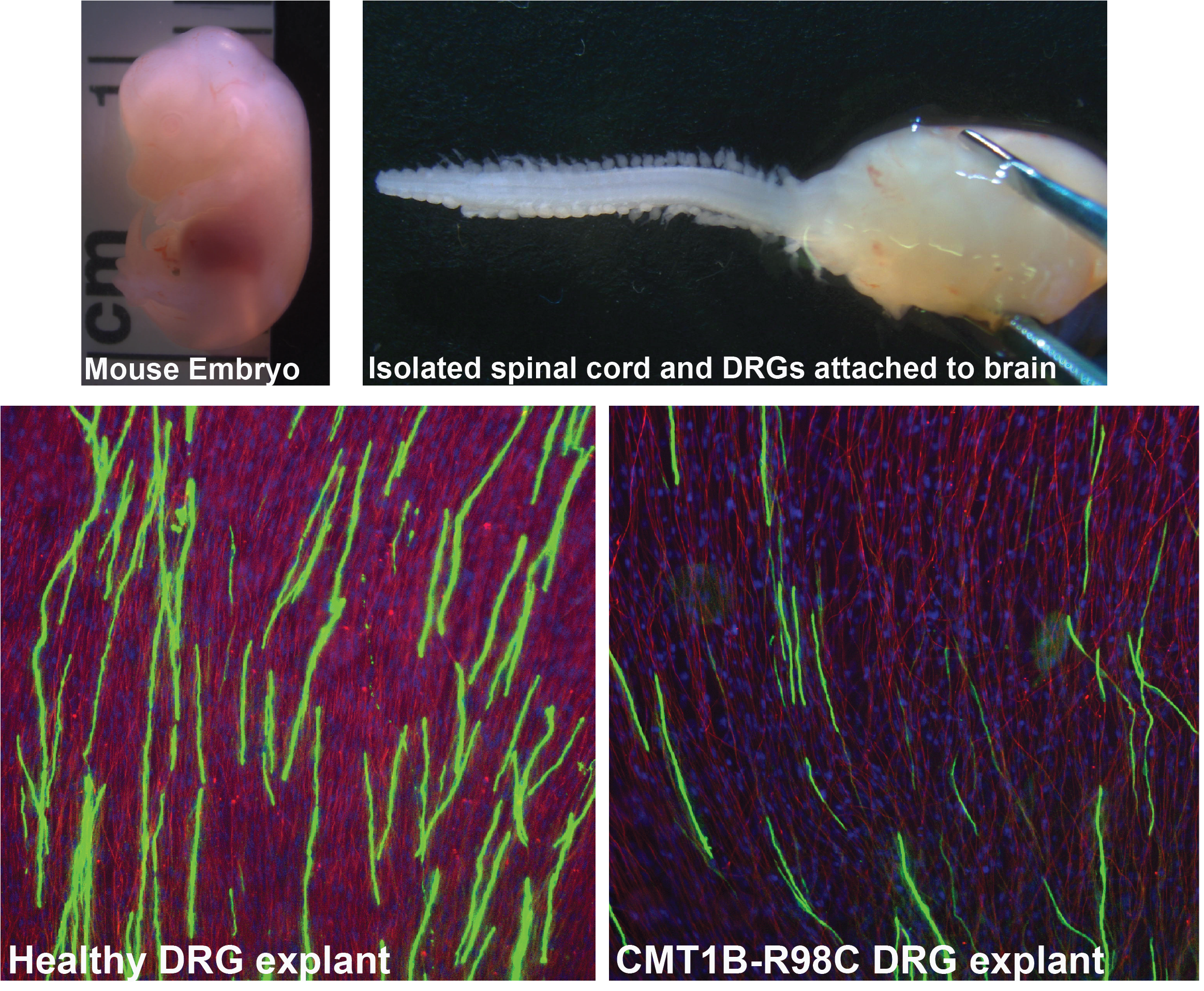
This figure shows the DRGs isolated from Healthy and CMT1B embryos and DRG explant culture as an ex vivo model of myelinating Schwann cells. This is a very useful model to studying myelination and diseasemechansim and to screen therapeutic componenets.
CMT1B is an inherited peripheral demyelination disorder caused by mutations in the MPZ gene. Over 200 different mutations have been identified in the MPZ gene, which are primarily autosomal dominant, resulting in the accumulation of mutant MPZ within Schwann cells, leading to peripheral nerve demyelination. Our research group is dedicated to understanding how reducing mutant MPZ aggregates within Schwann cells can promote remyelination. Our ultimate objective is to develop a mutation-independent gene therapy for CMT1B, applicable to a wide range of CMT1B patients.
We're using different methods to modify the capsids of AAV vectors. Our goal is to create novel AAV vectors that can specifically target Schwann cells.
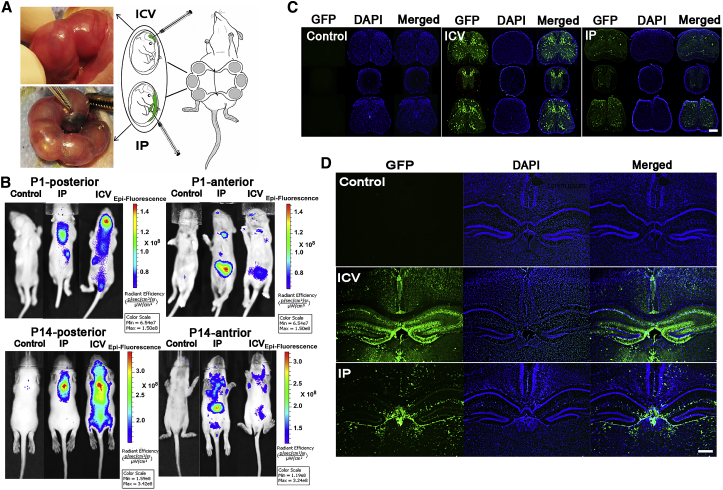
Prenatal (aka as in-utero or fetal) gene therapy not only provides a critical opportunity to address irreversible damage from inherited diseases before birth but also offers practical advantages. It requires a smaller amount of therapeutic AAV vector, making it potentially more cost-effective compared to postnatal gene therapy. Furthermore, because the therapy is initiated before the immune system is fully developed, there's a potential for immune tolerance to the transgene, enhancing the long-term efficacy of treatment. These combined benefits highlight the immense potential of prenatal gene therapy in revolutionizing the management of genetic disorders. The Rashnonejad Lab is working to improve this delivery method and explore its use for treating inherited diseases in utero.
Inside the Rashnonejad Lab
Our Research Interests
The team’s main research interests are:
- Developing gene therapies for inherited pediatric disorders
- Developing gene therapies for peripheral neuropathies
- Engineering AAV vectors and selective targeting of these vectors to the central and peripheral nervous systems
- Developing in-utero (aka prenatal or fetal) gene therapies
Featured Publications
Dr. Rashnonejad in the Media
Join Our Team!
We are committed to mentoring the next generation of scientists. Rashnonejad Lab is always seeking talented and passionate scientists to join the team, from students to postdocs to staff scientists who thrive in a fast-paced challenging research environment. If you have a desire to make a significant impact in rare pediatric neuromuscular and neurological diseases, please reach out to us at Afrooz.Rashnonejad@NationwideChildrens.org. Please include a cover letter explaining your research interests and future goals, along with your CV.
Connect With Dr. Rashnonejad
Are you interested in collaborating with the team at the Rashnonejad Lab? Complete the form below to connect.