Mann Lab
Approximately 20-30% of children with chronic kidney disease have an underlying genetic cause of their disease. Our lab is interested in better understanding the genetic landscape of kidney disease and the mechanisms by which these genetic changes affect kidney development and function.
We apply human genetics and basic cell biology techniques to study how genetic changes impact kidney function. Dr. Mann also co-directs the Kidney Genetics Clinic at Nationwide Children's Hospital, and we integrate clinical genetics with basic research with the goal of improving diagnosis, care and future treatments for children with inherited kidney conditions.
Featured Publications
- Mechanisms of Podocyte Injury in Genetic Kidney Disease
- Genetic Contributions to Lower Urinary Tract Dysfunction
- Mutations in PRDM15 Are a Novel Cause of Galloway-Mowat Syndrome
- CAKUT and Autonomic Dysfunction Caused by Acetylcholine Receptor Mutations
- Whole-Exome Sequencing Enables a Precision Medicine Approach for Kidney Transplant Recipients
Join Us!
The Mann Lab is actively recruiting undergraduate and post-doctoral researchers. If you are interested in joining our team or collaborating, please email Nina Mann at Nina.Mann@NationwideChildrens.org.
Meet Our Team

Nina Mann, MD
Principal Investigator
Nina.Mann@NationwideChildrens.org
Nina Mann, MD, earned her medical degree at Harvard Medical School before completing pediatrics residency and pediatric nephrology fellowship at Boston Children’s Hospital. During her training, she developed a strong interest in the genetic causes of childhood kidney disease. She now leads a research laboratory at Nationwide Children’s Hospital and The Ohio State University College of Medicine, where her group uses genomic and functional approaches to discover new disease genes and understand how genetic changes affect kidney development and function. Clinically, she directs the Kidney Genetics Clinic at Nationwide Children’s Hospital, integrating research discoveries into patient care. Outside of the lab, she spends time at her second job, personal assistant to two toddlers.

Wafaa Albalawy, MS, PhD
Postdoctoral Scientist
Wafaa.Albalawy@NationwideChildrens.org
Wafaa Albalawy, PhD, earned her bachelor’s degree in biochemistry from King Abdulaziz University in Saudi Arabia and a master’s degree in biomedical science from Hood College in Maryland. She then joined the Cancer Genomics Center at King Faisal Hospital and Research Center in Riyadh, Saudi Arabia where she contributed to molecular oncology research. Wafaa completed her PhD in human genetics at the University of Pittsburgh under the mentorship of Dr. Ora Weisz; her dissertation studied the direct effects of SGLT2 inhibitors on proximal tubule function. In July 2025, she joined Nationwide Children’s Hospital where her research focuses on elucidating the role of WT1 in kidney development and disease. Outside of the lab, Wafaa enjoys hiking, traveling, road trips and exploring new coffee spots.

Melisa Bilgin, MS
Research Associate
FerideMelisa.Bilgin@NationwideChildrens.org
Melisa Bilgin, MS, is a research associate at Nationwide Children’s Hospital, where she investigates how alternative splicing of the WT1 gene regulates kidney development and podocyte function. Her work uses iPSC-derived kidney organoids, proteomics and gene editing to uncover molecular mechanisms underlying pediatric kidney disease. Before joining the Mann Lab, she worked at The Ohio State University studying Fc receptor biology and immune modulation in cancer, and previously conducted her master’s research at the TUBITAK Genetic Engineering and Biotechnology Institute, developing nanoceria-based therapies for lung cancer. Melisa holds a master’s degree in molecular and translational biomedicine from Acibadem University. She is passionate about exploring how molecular mechanisms translate into functional biology and enjoys mentoring students in the lab. Outside the lab, she enjoys playing electric guitar and piano, discovering new restaurants and spending time with her family.
Research Projects
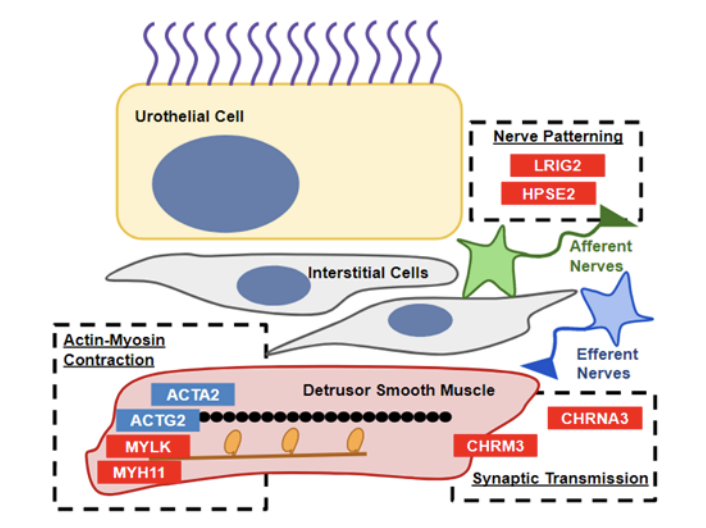
Lower urinary tract dysfunction can cause significant distress for patients. Our previous studies discovered that mutations in an acetylcholine receptor, CHRNA3, can cause recurrent urinary tract infections and bladder dysfunction. We are applying genomic tools to discover novel genetic causes for lower urinary tract dysfunction and to understand the functional consequences of these genetic changes.
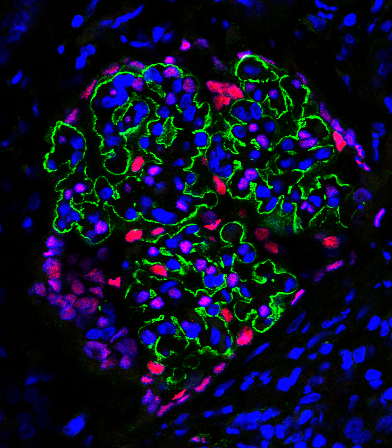
WT1, or wilms tumor 1, is a protein that plays an important role in kidney development and health, and mutations in WT1 can cause a spectrum of diseases, including wilms tumor, proteinuria and end-stage kidney disease. We are interested in better understanding the clinical spectrum of WT1-related disease as well as the mechanisms by which patient mutations may impact WT1 function.



