Search Results
Viewing: 1151-1160 of 11816 | All
Article
Hip Pointers
The term “hip pointer” is often used as a catch all phrase for any injury resulting in pain to the front of the hip. However, this is not always the case.

Article
How to Choose Running Shoes
Shoes that are chosen specifically for foot type and fitted properly can help keep athletes healthy and possibly prevent injuries such as shin splints and stress fractures.

Article
Elbow Injuries in Young Throwers
With the increasing participation and competitive level of today’s youth sports, more adolescent and pediatric patients are being evaluated and treated for a variety of elbow injuries.
Article
Game Day Fueling Plan for Athletes
The following are suggestions from Nationwide Children's Hospital on what a young athlete should eat and drink before, during and after a sporting event.
Article
Highlights of the OHSAA Concussion Policy
Are you familiar with the OHSAA concussion policy? The policy states that all adults involved with high school athletics are responsible for knowing, understanding, and following this policy.

Article
Salivary Gland Ablation
Prior to advancements at Nationwide Children’s Hospital, there were no successful interventional radiological (minimally invasive) therapies for ranulas and sialorrhea. The need for salivary gland ablation treatment was recognized, so our interventional radiologists developed a new procedure.
Article
Sclerotherapy of Orbital Slow Flow Malformations: A 15 year Single Center Experience
Authors James Murakami Daniel Straka Jeremy Tan Amanda Gibson Jill Foster Cameron Nabavi Craig Czyz Kenneth Cahill Purpose Present our core techniques for sclerotherapy of orbital slow-flow malformations Present our 15 year experience with sclerotherapy of orbital slow-flow vascular malformations
Multimedia
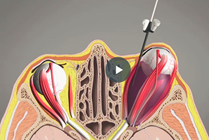
Large Cyst Treatment
For large cysts, a catheter can be placed entirely within the cyst to completely decompress it and temporarily fill it with sclerosant(s) that will be entirely aspirated prior to the end of the procedure. For referrals within the United States, please click the Request an Appointment link below.
Multimedia
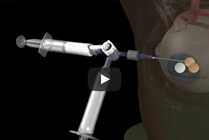
Small Cyst Treatment
For smaller cysts and even relatively solid disease it is better to aspirate with a small needle and then inject an agent such as Doxy or Bleo foam that you intend to leave in the lesion. For referrals within the United States, please click the Request an Appointment link below.
