Conditions
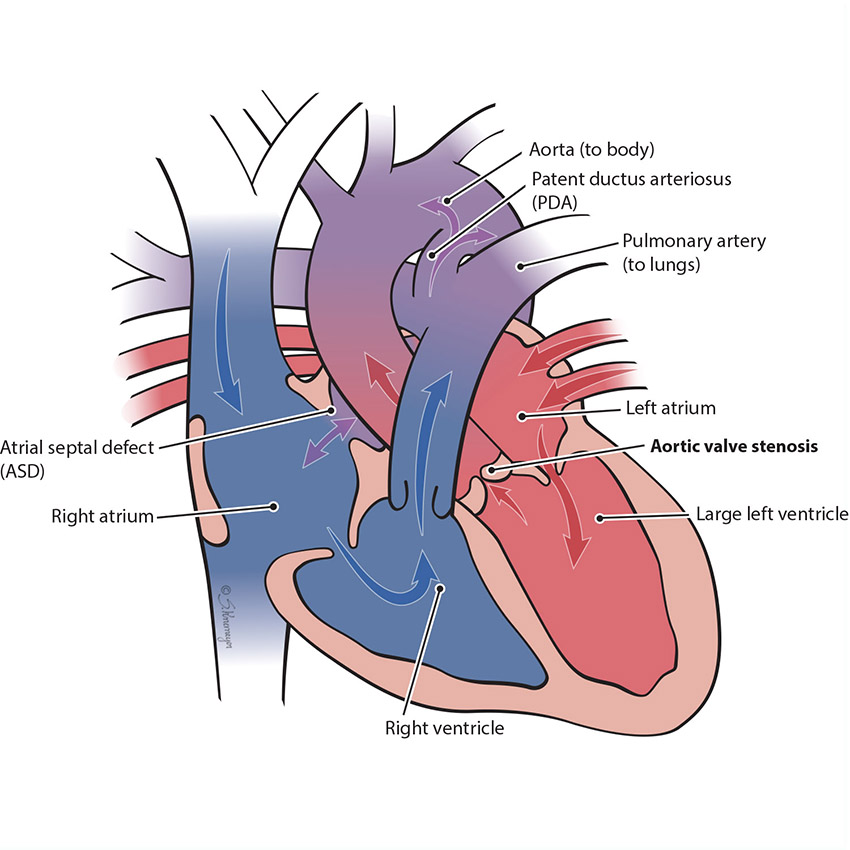
In the normal heart, blood flows from the left ventricle through the aortic valve to the rest of the body. Aortic valve stenosis is when the aortic valve is more narrow than normal and does not open properly. This blocks flow to the body. Aortic stenosis can be mild, moderate or severe. When a baby has severe aortic stenosis, the left pumping chamber (left ventricle) gets enlarged and weak.
Treatment for Aortic Stenosis
Mild and moderate aortic stenosis often does not need treatment. If the aortic stenosis progresses to severe, then treatment to improve the body blood flow will be needed.
Treatment choices include:
- Balloon dilation or valvuloplasty. This procedure is done as a cardiac catheterization. A heart catheterization uses a thin, flexible tube (catheter) with a balloon inside of it. The catheter is inserted into the leg blood vessel and passed up to the heart. When the balloon-tipped catheter reaches the narrowed aortic valve, the balloon is inflated to stretch the valve open.
- Surgery. Open heart surgery may be necessary to open the valve.
- Many babies with severe aortic stenosis also have other issues in the heart. The left ventricle and mitral valve can be affected and multiple complex procedures may be required.
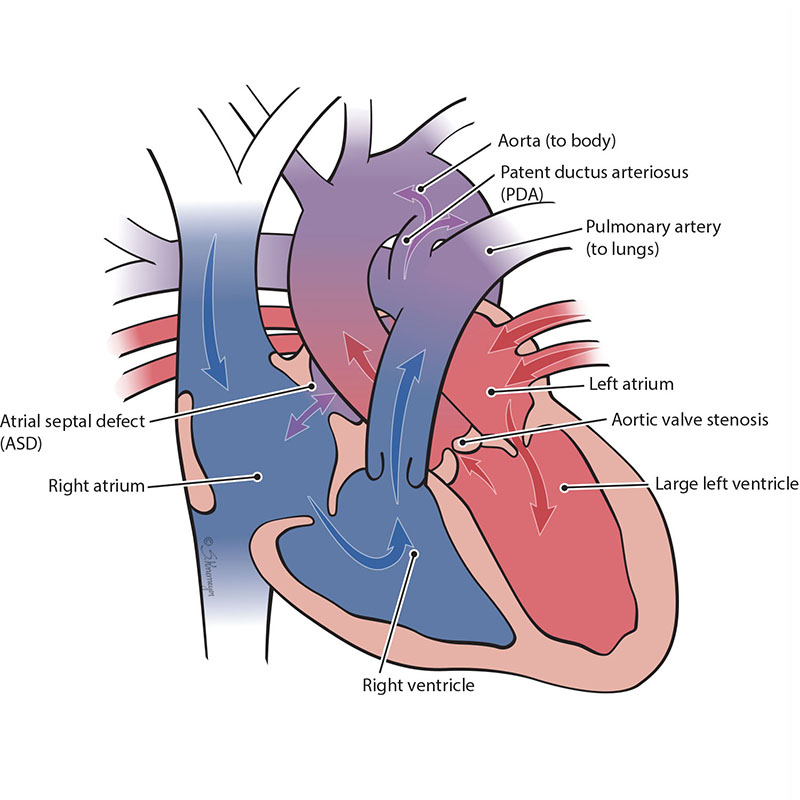
In the normal heart, blood flows from the left ventricle through the aortic valve to the rest of the body. Aortic valve stenosis is when the aortic valve is more narrow than normal or blocked. This blocks blood flow to the body. Aortic valve stenosis can be mild, moderate, or severe. When a baby has severe aortic stenosis, the left pumping chamber (left ventricle) gets enlarged and weak. This makes less blood flow through the left heart, which causes the left ventricle to stop growing and the left heart to be too small. Aortic stenosis can but doesn’t always cause a condition called hypoplastic left heart syndrome (HLHS).
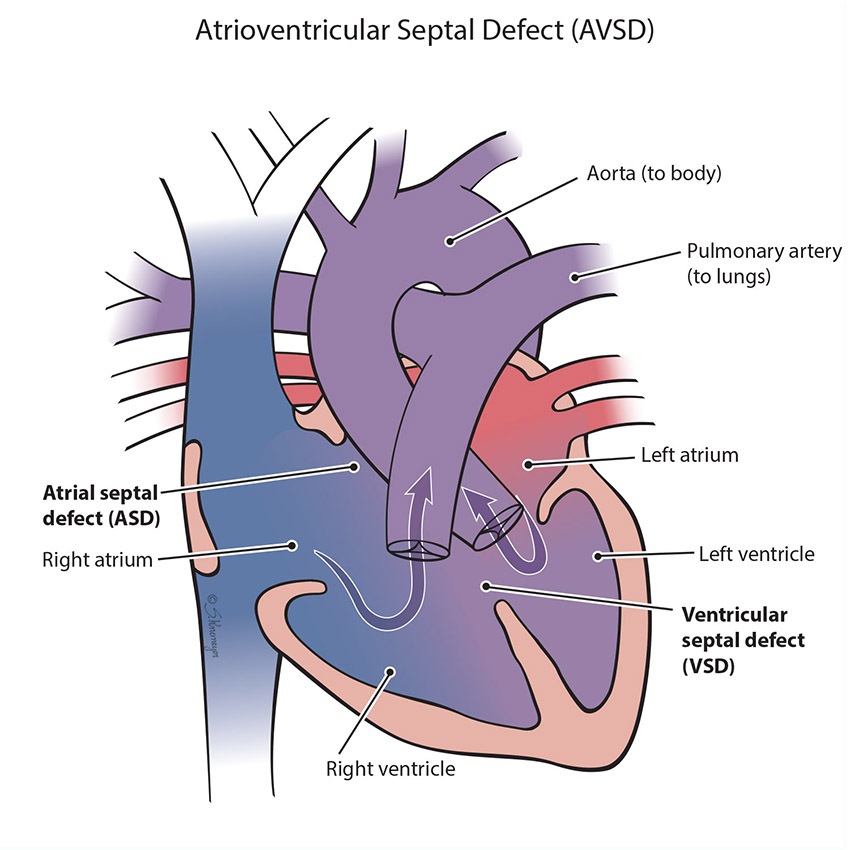
An atrioventricular septal defect (AVSD) is a heart defect in which there is a hole between both the top collecting chambers (atria) and bottom pumping chambers (ventricles). The valves between the chambers (the tricuspid and the mitral valves) are involved in the hole and do not form separately. Instead, one large valve is formed. Blood can flow across the holes and cause extra blood flow to the lungs. There are multiple forms of AVSD – partial, complete, transitional. In a complete AVSD, the hole in the center of the heart is large. In partial and transitional types the hole is smaller. This is seen more commonly in children with Down syndrome, but happens frequently in other children as well.
Treatment for AVSD
All forms of AVSD will need surgical repair at some point with open heart surgery. Large holes need surgery early, in the first year, but other types can be done later. During surgery, any holes in the chambers are closed using patches. The valves usually require repair during surgery. Closing the holes will prevent lung damage from the extra blood flow to lungs across the holes. Your baby’s cardiologist will decide the best time for surgery based on your baby’s specific heart defect.
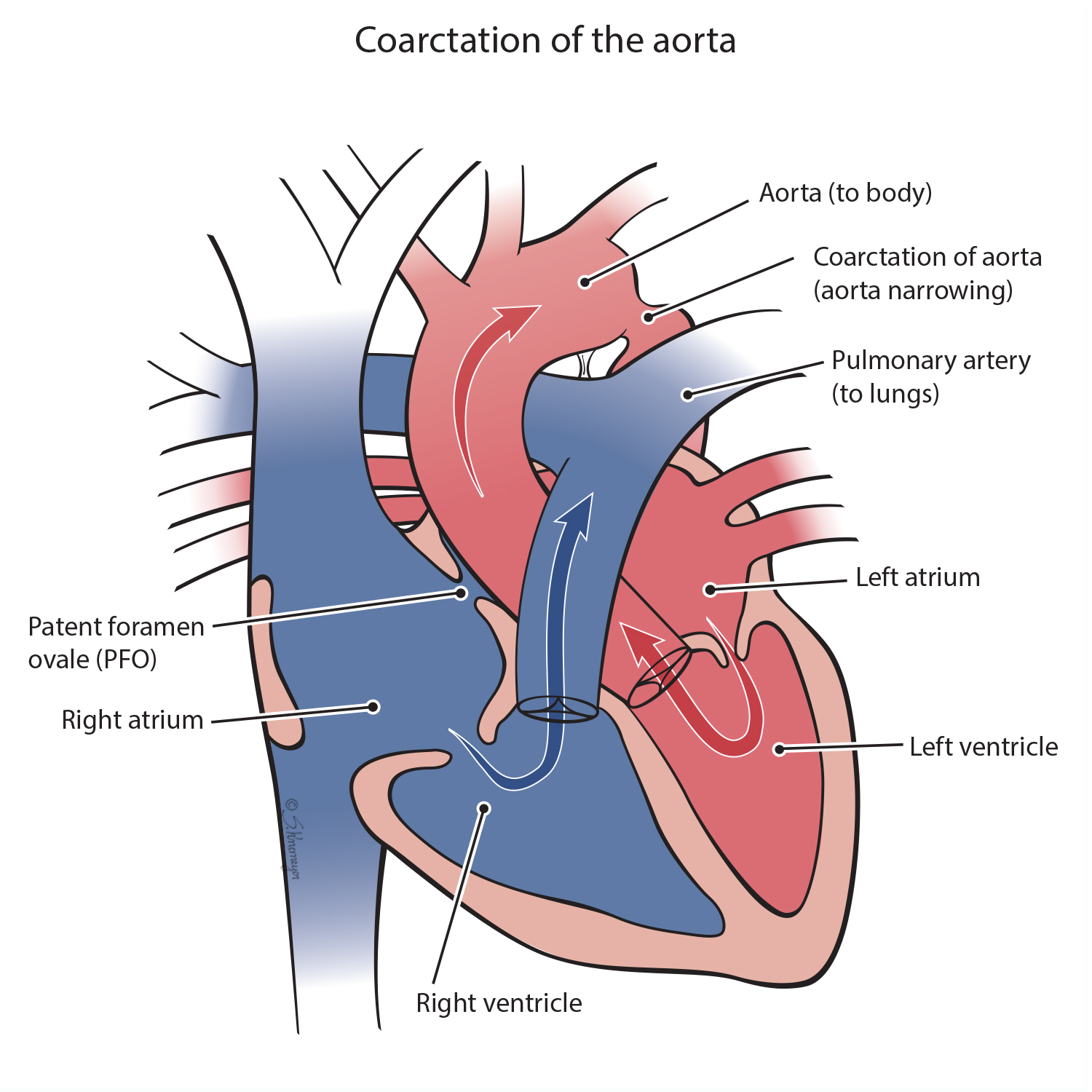
A coarctation of the aorta is a narrowed part of the aorta, the large vessel that carries blood to the body. The narrowing causes decreased blood flow to the lower body. The left ventricle of the heart must work harder in order to pump blood through the narrowed aorta. A “critical” coarctation means the aorta is so narrowed that blood cannot flow out to the body.
Treatment for Coarctation of the Aorta
Most children with an aortic coarctation, unless very mild, will need repair soon after birth. Coarctation of the aorta can be treated either with surgery or a cardiac catheterization (“heart cath”). The choice for surgery or catheterization is dependent on the age of the child and the exact details of the heart defect. While older children and young adults can be treated with a catheterization, most young children will need surgery.
During surgery the narrow portion of the aorta is removed, and the aorta is patched and reconnected. This opens the narrowing, fixing the blood flow through the vessel to the lower half of the body, though more treatments may be needed over time.
A heart catheterization uses a thin, flexible tube, called a catheter, which has a balloon and/or stent inside it. The catheter is inserted into the leg blood vessel and passed up to the narrow part of the aorta. When the catheter reaches the narrow area of the aorta, a balloon is inflated to open the narrowing. Sometimes a stent is required to keep the vessel open. Both the balloon and stent treatments are effective, but more treatments may be needed over time.
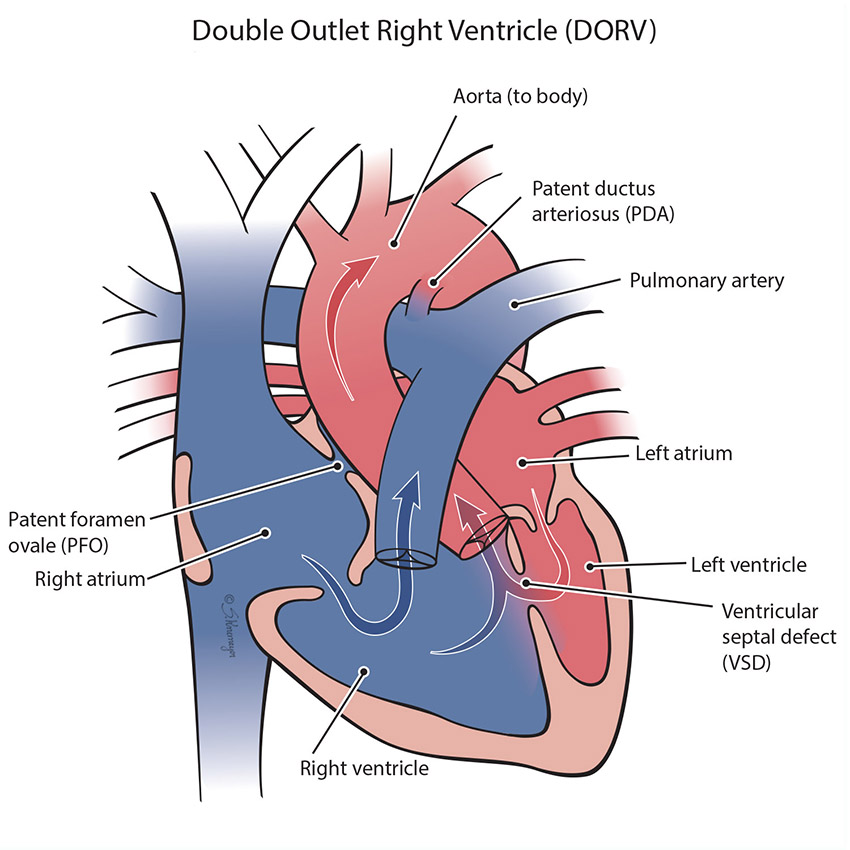
In a normal heart, the aorta comes off the left pumping chamber (ventricle) and the pulmonary artery comes off the right pumping chamber (ventricle). In DORV both the pulmonary artery and the aorta (the “outflow vessels”) come from the right ventricle. This defect usually also has a large ventricular septal defect (VSD), a hole between the bottom chambers. DORV frequently will have additional heart defects and is very unique to each child.
Treatment for Double Outlet Right Ventricle (DORV)
All forms of DORV will need some form of heart surgery to correct the normal flow of blood. Most forms of DORV are able to have a “complete repair”, where the blood flow is restored back to normal and the hole in the bottom chambers (VSD) is closed. The timing of surgery will depend on the exact anatomy of the DORV, but can vary from the newborn period to 1 year of age. Some infants with DORV will need more than one surgery.
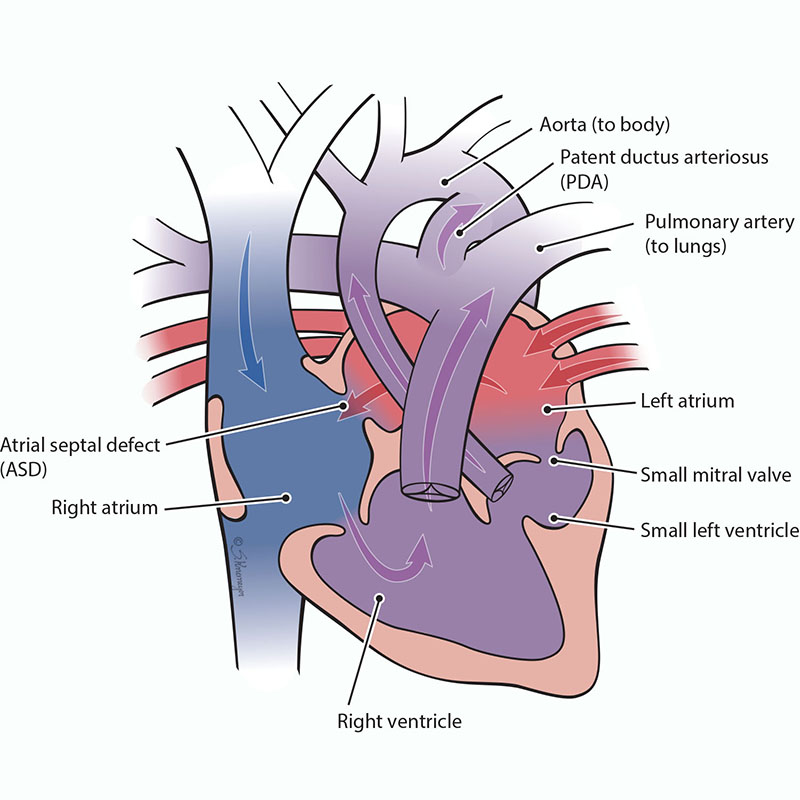
In DORV, both the pulmonary artery and the aorta start in the lower right pumping chamber (right ventricle). This defect often has a ventricular septal defect (VSD), pulmonary stenosis, or a transposition of the great arteries as well. A VSD is a hole between the bottom chambers. Pulmonary stenosis is when the pulmonary valve is too small. Transposition of the great arteries is when the aorta and pulmonary artery positions are switched. When DORV is combined with a too small (hypoplastic) left ventricle, this heart condition is known as a single ventricle defect.
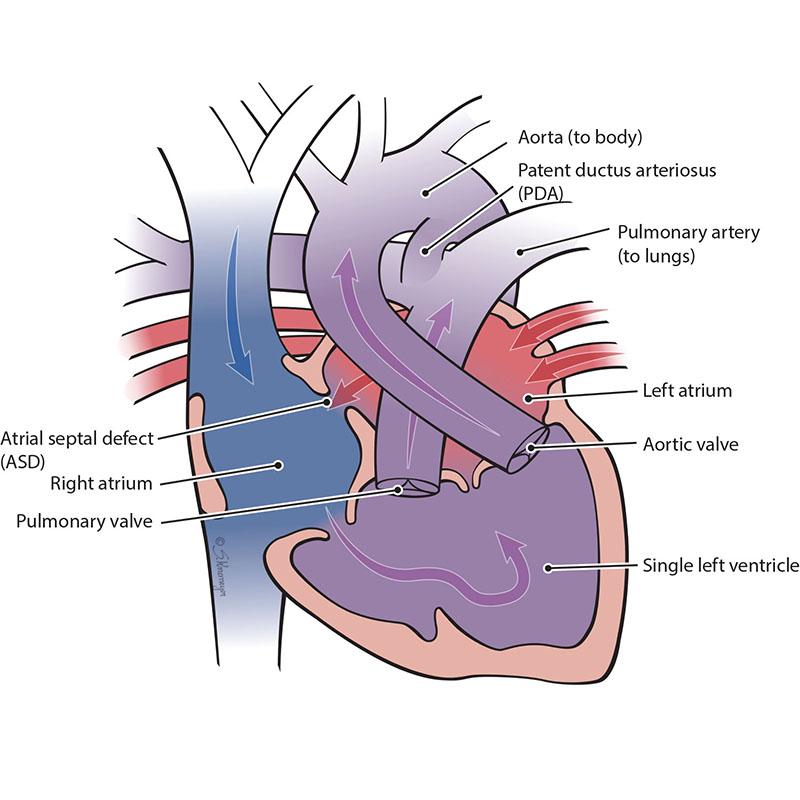
Double-inlet left ventricle is a condition when only the left lower pumping chamber (left ventricle) is fully grown (developed). The chamber that collects blood from the lungs (the left atrium) and the chamber that collects blood from the body (the right atrium) both send blood into a single left ventricle. The blood from the left ventricle is then pumped out of the heart through the pulmonary artery and aorta to the lungs and body at the same time. However, because all of the blood flowing through the heart mixes in the left ventricle, both red (oxygenated) blood and blue (deoxygenated) blood mixes together before going out to the body.
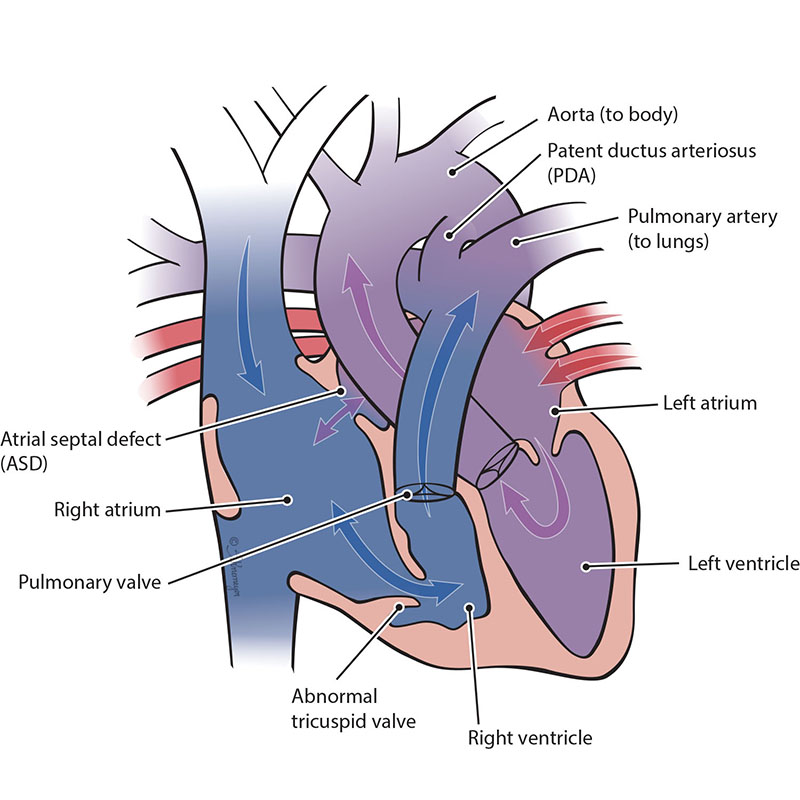
Ebstein’s anomaly is a heart defect where the tricuspid valve - the valve between the two right heart chambers (right atrium and right ventricle) - doesn’t work properly or is malformed. Blood may leak back through the valve, making your baby’s heart work harder. Ebstein’s anomaly may also lead to enlargement of the right side of the heart or heart failure. Many babies with severe Ebstein’s need single ventricle surgeries.
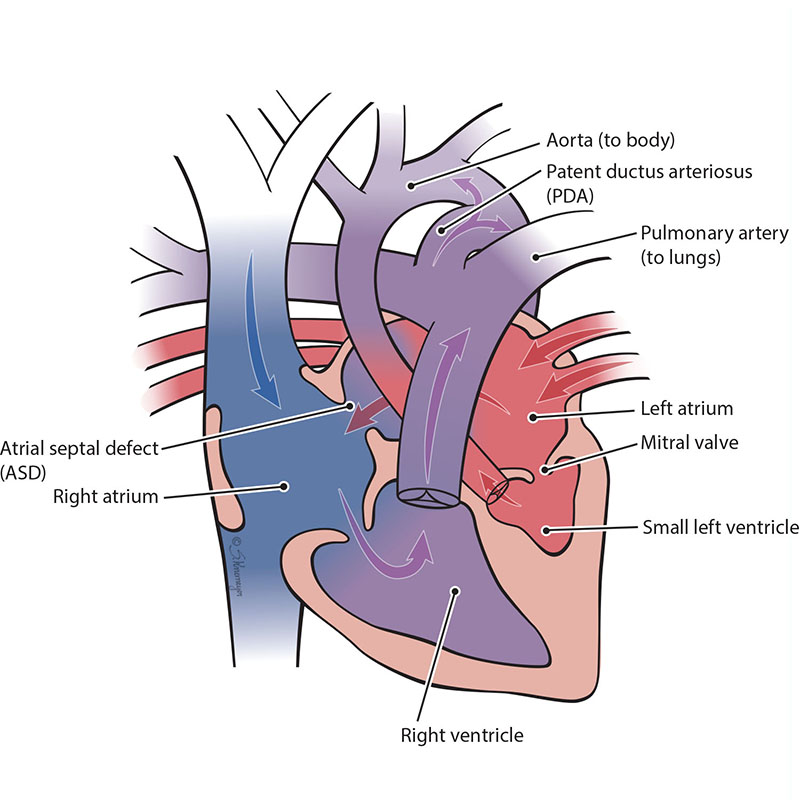
In HLHS, the parts on the left side of the heart (mitral valve, left ventricle, aortic valve and aorta) do not develop all the way and are too small (hypoplastic). When the left lower pumping chamber (left ventricle) is hypoplastic, it cannot get enough oxygenated blood to the body. When a baby has HLHS, the heart needs a connection between the aorta and pulmonary artery to get the oxygenated blood out to the body and brain. This means the baby needs to have an atrial septal defect (ASD) and a patent ductus arteriosus (PDA) to live.
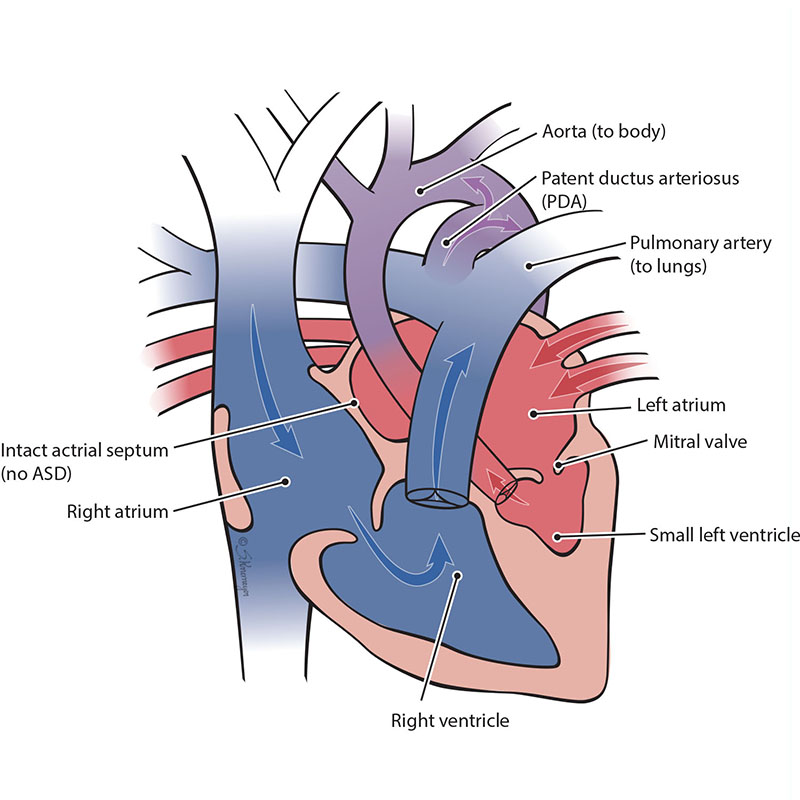
In rare cases, an unborn baby with severely underdeveloped, left-sided heart chambers (hypoplastic left heart syndrome or ‘HLHS’) can have a blockage within the heart. This is called HLHS with an intact atrial septum. An intact atrial septum is when the wall between the left and right upper chambers of the heart (atria) is closed. Having both HLHS and an intact atrial septum can make the baby very sick right after birth. The blood returning from the lungs into the left upper chamber (left atrium) cannot get out of the chamber. This is because the left side of the heart did not grow (develop) and the atrial septum is closed. This makes blood backup in the lungs, causing lung damage.
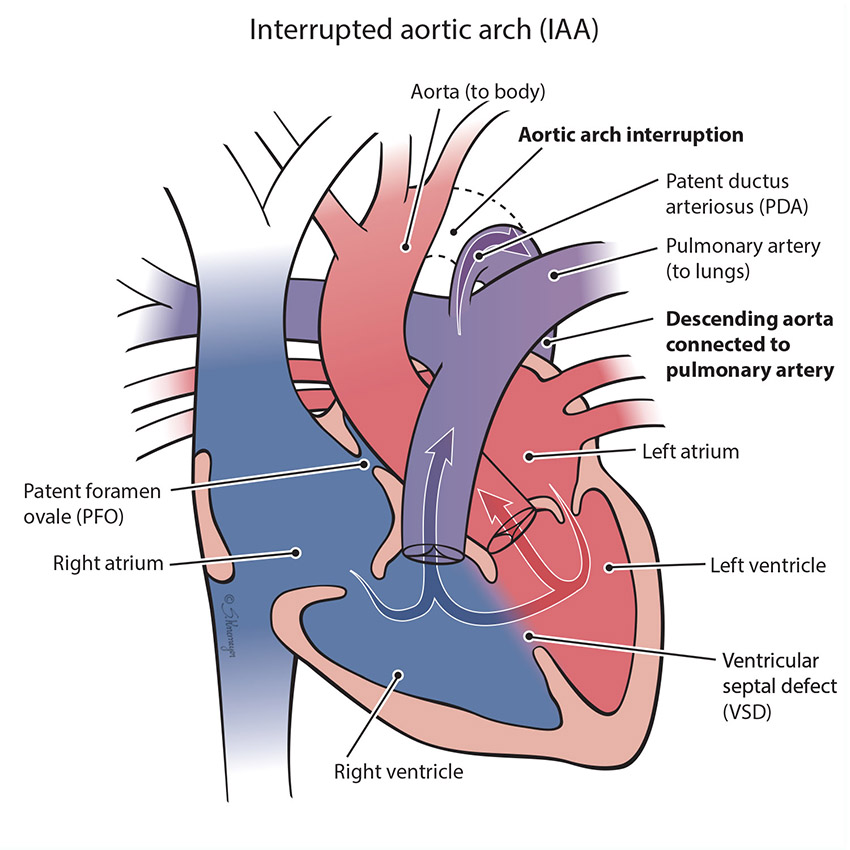
The aorta is the large vessel that carries blood from the left ventricle out to the body. An interrupted arch happens when the aorta does not form correctly - a section of aorta is missing so that the part coming off the heart and the part going out to the body are not connected. When this happens, blood cannot get from the heart to the lower body without an extra blood vessel called a patent ductus arteriosus (PDA). IAA usually involves a hole in the lower heart chambers called a ventricular septal defect (VSD).
Treatment for Interrupted Aortic Arch
All forms of interrupted arch will need surgical repair (open heart surgery) soon after birth, typically done in the first week. This heart defect is most commonly found in the newborn period, when the baby is small, so sometimes more than one surgery is needed. The surgery will connect the two parts of the aorta together, and close any large holes (VSD) if present. The aorta repair can be a direct connection of the two parts, or sometimes artificial tissue will need to be placed if the gap is large.
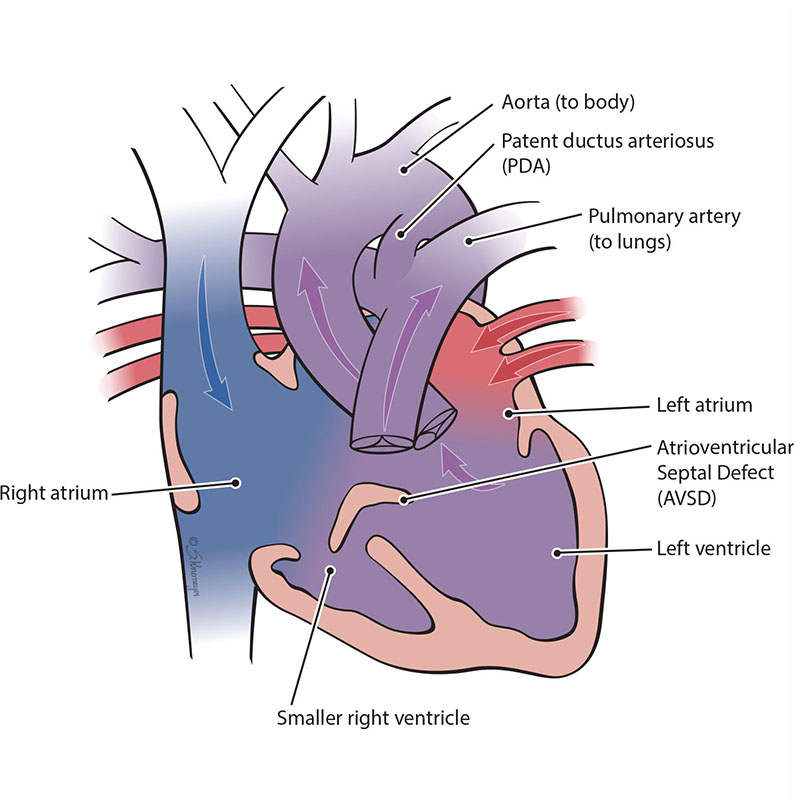
An atrioventricular septal defect (AVSD) is a heart defect where there are holes between both the top collecting chambers (atria) and bottom pumping chambers (ventricles). The valves between the atria and ventricles can be two separate valves or combined into one large valve. AVSD is also known as an atrioventricular canal defect (AVC or AVCD). In an unbalanced, left-dominant atrioventricular septal defect, the left ventricle is larger than the right ventricle. The difference in size between the ventricles can range from small to large. In severe cases, the right ventricle is too small to pump blood into the lungs.
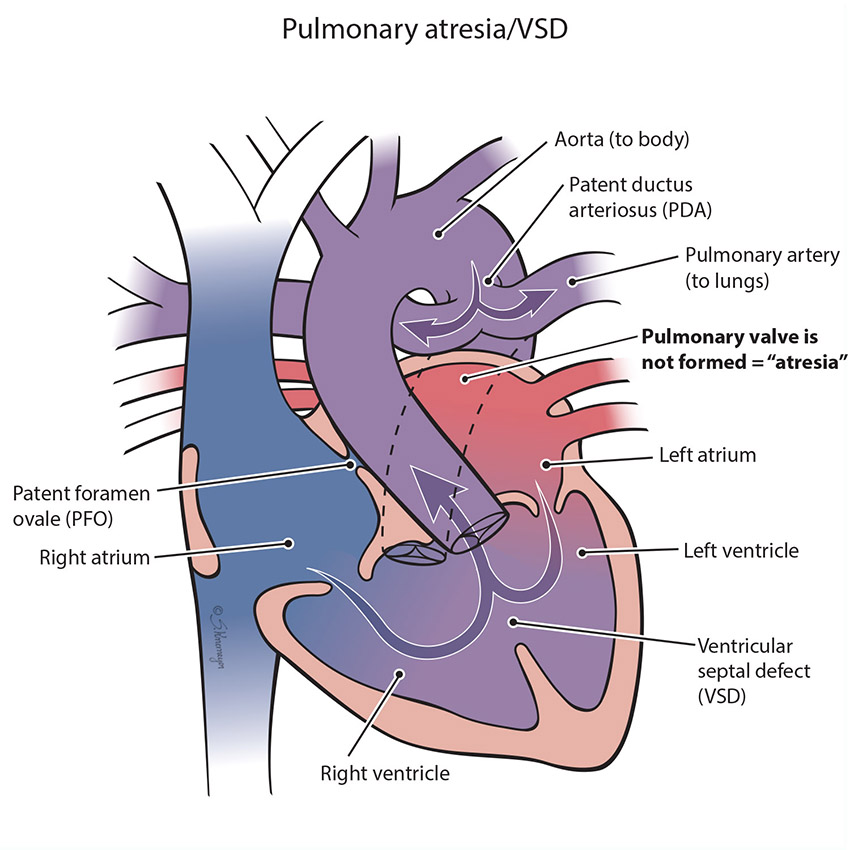
The pulmonary valve is a “door” that opens and closes between the right lower pumping chamber (the right ventricle) and the lungs. The condition known as pulmonary atresia happens when the pulmonary valve does not form, so that no blood can go from the heart into the lungs. When babies have this defect, blood cannot flow normally to the lungs to pick up oxygen for the body, and baby will have low oxygen levels. Immediately after birth an extra blood vessel known as the patent ductus arteriosus (PDA) will support the lung blood flow temporarily, but additional treatment is usually necessary.
Treatment for Pulmonary Atresia/VSD
Treatment for pulmonary atresia depends on its severity. Most babies born with pulmonary atresia need surgery to correct the problem soon after birth. The goal is to improve blood flow to the lungs, either by opening the narrowed pulmonary valve or bypassing it with an extra blood vessel called a “shunt” – this is a surgically placed tube that takes the place of the PDA. The surgeon will close the ventricular septal defect by using a patch when needed. Cardiac catheterization, a “heart cath,” is sometimes an option for newborns with pulmonary atresia/VSD. During this procedure the pulmonary valve is stretched open by using a balloon. In addition, sometimes a stent can be placed within the PDA to keep it open, so that a surgical shunt is not needed. Multiple heart procedures, a combination of both surgeries and catheterizations, are usually necessary throughout childhood for children pulmonary atresia/VSD.
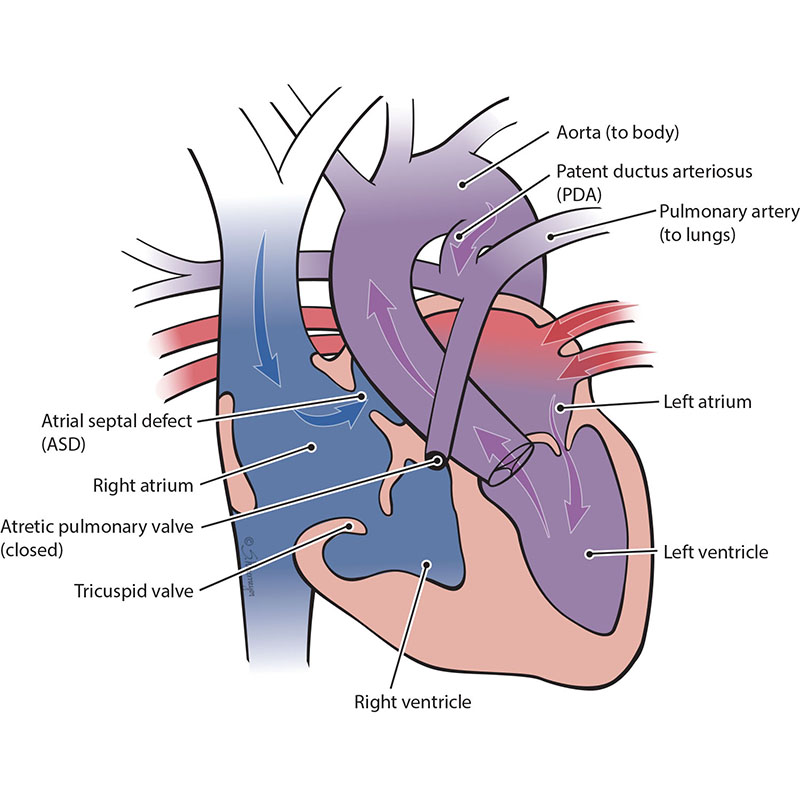
The pulmonary valve is a “door” that opens and closes between the right lower pumping chamber (the right ventricle) and the lungs. The condition known as pulmonary atresia with intact ventricular septum happens when the pulmonary valve stays closed and there is no hole between the bottom chambers of the heart. This means that blood cannot move from the right ventricle to the lungs like normal. When the right-sided chambers do not grow, this becomes a single ventricle defect. In this case, only the left ventricle is fully working.
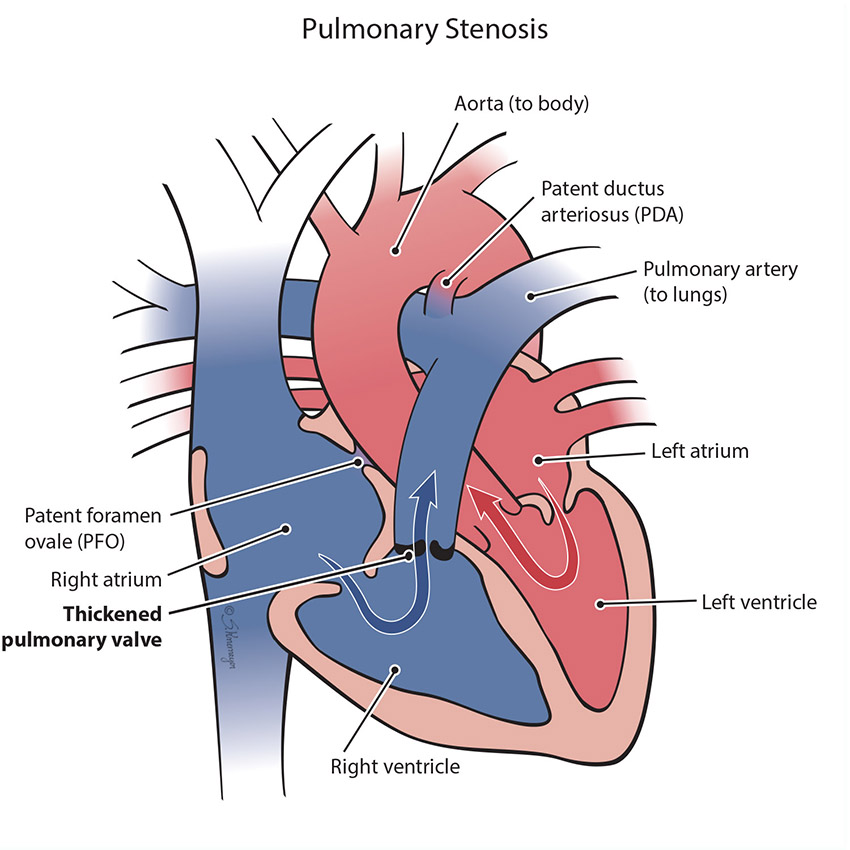
In the normal heart, blood flows from the right ventricle through the pulmonary valve to the lungs. Pulmonary stenosis is when the pulmonary valve is more narrow than normal and does not open properly. This makes it harder for the right ventricle to pump blood to the lungs. If severe, the blood cannot be pumped into the lungs well and the baby may have low oxygen levels.
Treatment for Pulmonary Stenosis
Mild and moderate pulmonary stenosis often does not need treatment. If the pulmonary stenosis progresses to severe, then treatment to improve the lung blood flow will be needed.
Treatment choices include:
- Balloon dilation or valvuloplasty. This procedure is done as a cardiac catheterization. A heart catheterization uses a thin, flexible tube (catheter) with a balloon inside of it. The catheter is inserted into the leg blood vessel and passed up to the heart. When the balloon-tipped catheter reaches the narrowed pulmonary valve, the balloon is inflated to stretch the valve open.
- Surgery. Open heart surgery may be necessary to open the valve and the lung arteries. Sometimes a “pulmonary valve replacement,” a new pulmonary valve, can be placed.
- Most children are able to have an initial catheterization / balloon procedure, although if the stenosis progresses, additional surgery later on may become necessary.
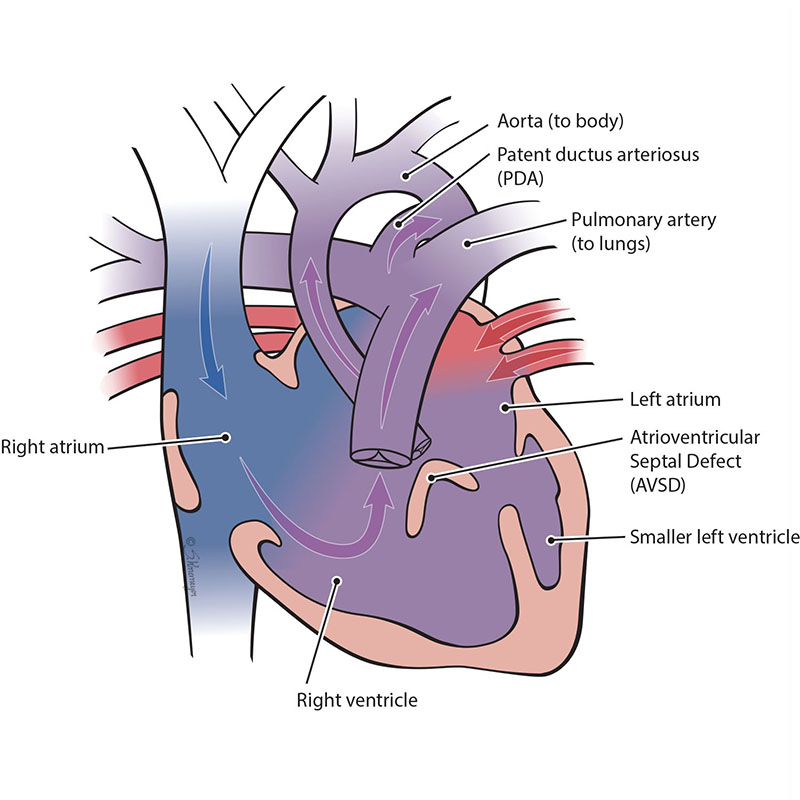
An atrioventricular septal defect (AVSD) is a heart defect where there are holes between both the top collecting chambers (atria) and bottom pumping chambers (ventricles). The valves between the atria and ventricles can be two separate valves or combined into one large valve. AVSD is also known as an atrioventricular canal defect (AVC or AVCD).
In an unbalanced, right-dominant atrioventricular septal defect, the right ventricle is larger than the left ventricle. The difference in size between the ventricles can range from small to large. When the left ventricle is too small, it cannot pump blood out to the body and brain. This heart condition is known as a single ventricle defect.
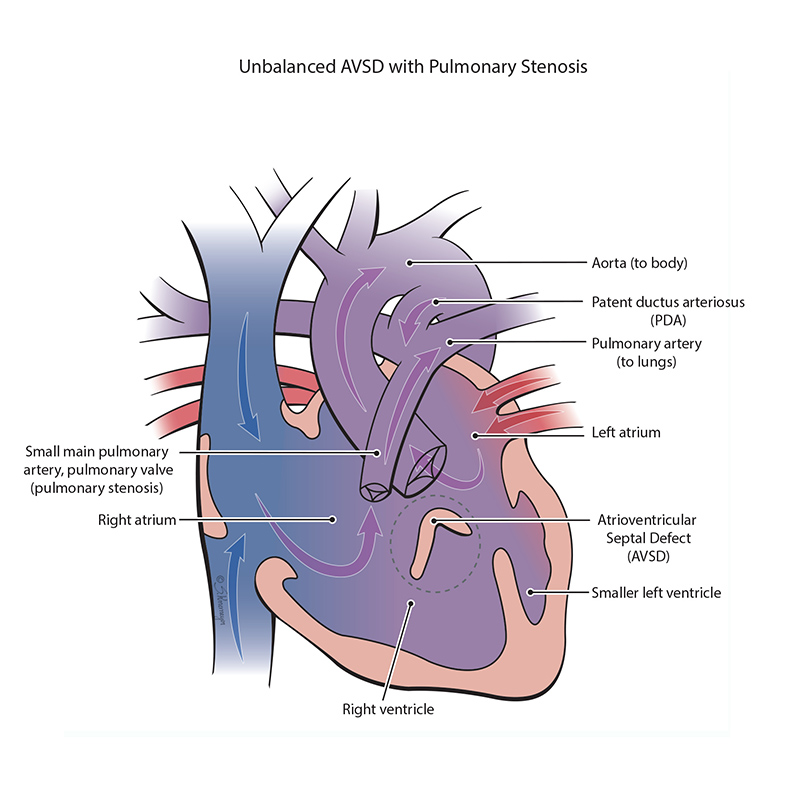
An atrioventricular septal defect (AVSD) is a heart defect where there are holes between both the top collecting chambers (atria) and bottom pumping chambers (ventricles). The valves between the atria and ventricles can be two separate valves or combined into one large valve. AVSD is also known as an atrioventricular canal defect (AVC or AVCD).
In an unbalanced, right-dominant atrioventricular septal defect, the right ventricle is larger than the left ventricle. The difference in size between the ventricles can range from small to large. When the left ventricle is too small, it cannot pump blood out to the body and brain. This heart condition is known as a single ventricle defect. Some combinations of heart defects may include pulmonary stenosis. Pulmonary stenosis is when the pulmonary valve is more narrow than normal and does not open properly. When there is pulmonary stenosis in combination with AVSD, this can be protective, balancing the amount of blood sent to the lungs and the body. However, if the pulmonary stenosis is severe, the blood cannot be pumped into the lungs well and the baby may have low oxygen levels.
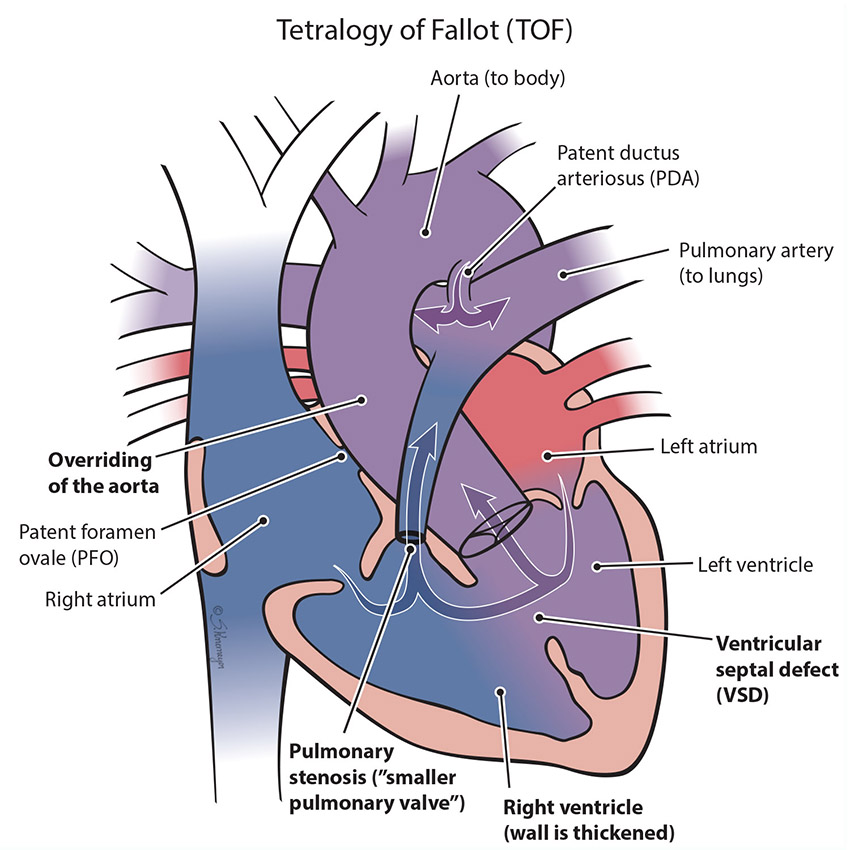
Tetralogy of Fallot (TOF) is a combination of 4 congenital heart problems that result in not enough blood flow to the lungs. In this condition, severity can range from mild to severe. These 4 heart problems are:
Ventricular septal defect (VSD): A hole between the 2 bottom pumping chambers of the heart (ventricles).
Overriding of the aorta: In the normal heart, the aorta comes off the left ventricle. In TOF, it sits over both the right and left ventricles and “straddles” the VSD.
Pulmonary stenosis: The pulmonary valve is more narrow than normal and does not open properly. This makes it harder for the right ventricle to pump blood to the lungs, and as a result baby may have low oxygen levels.
Right ventricular hypertrophy: Increased thickness of the walls of the right pumping chamber (ventricle) because the right heart has to work harder to pump blood to the lungs.
Treatment for Tetralogy of Fallot
All children born with Tetralogy of Fallot need to have surgery at some point. Most children will need surgery before they turn 1 year old, most commonly around 6 months of age. The goal for surgery is to improve blood flow to the lungs and the rest of the body. Some children will need a temporary shunt placed to provide stable pulmonary blood flow until a more permanent repair can be made at a later age. Surgical repair for TOF will fix the pulmonary stenosis by opening the pulmonary valve, and close the VSD using a patch. Another surgery later in life is sometimes necessary.
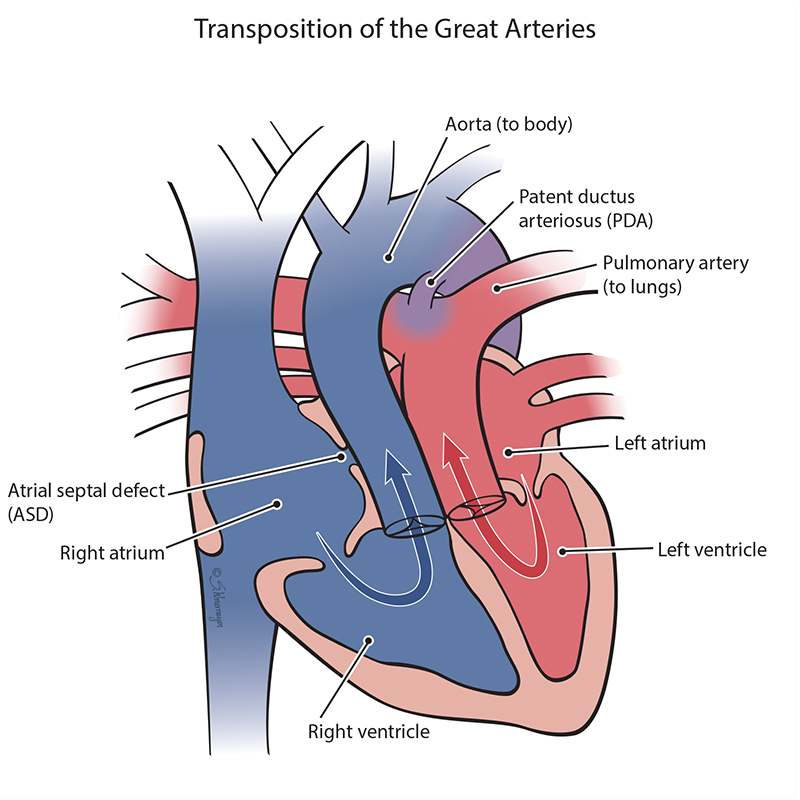
In a normal heart, oxygen-poor (“blue”) blood from the body returns to the right heart and is pumped through the pulmonary artery to the lungs to get more oxygen. Then, oxygen-rich (“red”) blood returns to the left heart and is pumped out to the body through the aorta. In transposition of the great arteries, the two major arteries leaving the heart (pulmonary artery and aorta) are switched and get blood from the wrong chamber. This means that the aorta is connected to the right ventricle, and the pulmonary artery is attached to the left ventricle. Most babies born with transposition of the great arteries have lower oxygen levels at birth and may have problems breathing.
In TGA, immediately after birth the “blue” blood and “red” blood need to mix to make sure enough oxygen gets to the body. Mixing happens two ways – within the top chamber hole (ASD) or through an extra blood vessel called the patent ductus arteriosus (PDA). If there is enough mixing, babies with TGA can be very stable after birth. However, babies with TGA who do not have enough mixing can have very low oxygen levels and require emergency treatment.
Treatment for TGA
All children born with TGA will need to have surgery to switch the blood vessels. Surgery is typically done in the first week of life once the baby is stable after birth. Immediately, they may need help with breathing, extra oxygen, or a medicine called prostaglandin E (PGE). This medicine keeps the blood flowing through the PDA. A balloon atrial septostomy (BAS) can be done shortly after birth by cardiac catheterization if needed. This uses a balloon on a catheter to enlarge the atrial septal defect (ASD), which increases mixing and makes it easier for oxygen-rich blood to reach the rest of the body.
The surgery for TGA repair is called an arterial switch. The goal is to restore the normal blood flow through the heart and out to the lungs and the body. The surgeon will move the aorta and pulmonary artery back to their normal places. The coronary arteries also need to be moved and reattached to the aorta.
The tricuspid valve is the “door” that opens and closes between the right upper collecting chamber (right atrium) and the right lower pumping chamber (the right ventricle). The condition known as tricuspid atresia is when the tricuspid valve does not grow (develop). This means blood cannot move from the right atrium to the right ventricle. When the right ventricle does not grow well, blood can only move into the right ventricle through a hole that is between the two pumping chambers. This hole is called a ventricular septal defect or VSD. Only the left ventricle develops to pump blood. This defect is sometimes called “hypoplastic right heart.” There are 2 main forms of tricuspid atresia depending on how the blood vessels connect, transposition of the great arteries or normally related great arteries.
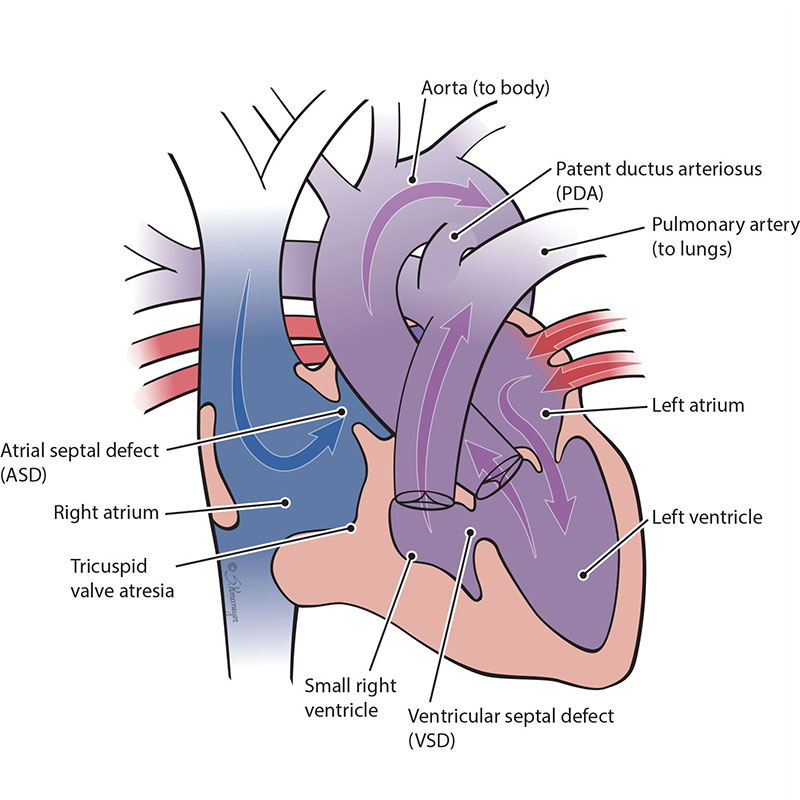
In Tricuspid Atresia with Normally Related Great Arteries, the aorta connects to the main left ventricle. The left ventricle pumps blood to the aorta (body) and through the hole (VSD) to the lungs (pulmonary).
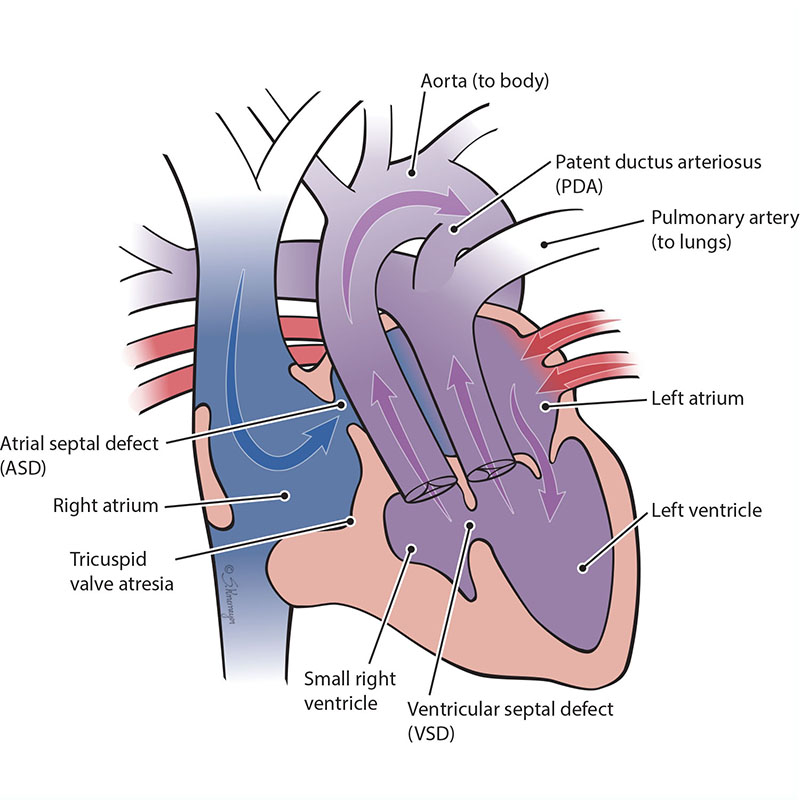
In Tricuspid Atresia with Transposition of the Great Arteries, the aorta connects to the small right ventricle. The body receives blood from the left ventricle through the VSD.
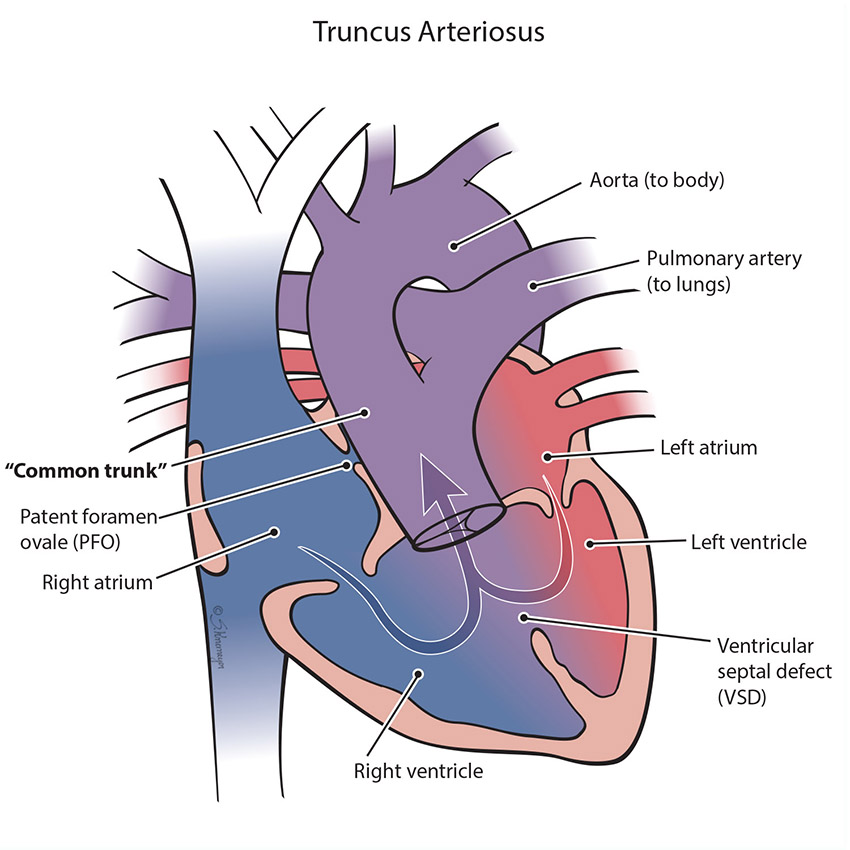
When a normal heart develops, there are two large blood vessels (great arteries) that take the blood to the body (aorta) and the lungs (pulmonary artery). Sometimes only one blood vessel forms instead of two. This is called a “common trunk,” or truncus arteriosus. With this defect, there is also a hole between the ventricles called ventricular septal defect (VSD). Blood “mixes” inside the heart through the VSD hole before getting pumped though one big blood vessel to both the lungs and the body. Because blood leaving the heart is “mixing,” baby may have lower oxygen levels and trouble breathing.
Treatment for Truncus Arteriosus
All children born with truncus arteriosus will need to have surgical repair to close the hole, and separate the “common trunk” into two separate blood vessels for the body and the lungs. The surgeon uses the following steps to repair the defect:
- Close the ventricular septal defect by using a patch.
- Detach the pulmonary arteries from the truncus arteriosus (common artery) and connect them to the right ventricle. This new connection requires a tube, either a homograft or a conduit. A homograft is a human tissue valve. A conduit is a small tube containing a valve. Because this tube does not grow with the child, it usually needs replacement at some point in childhood.
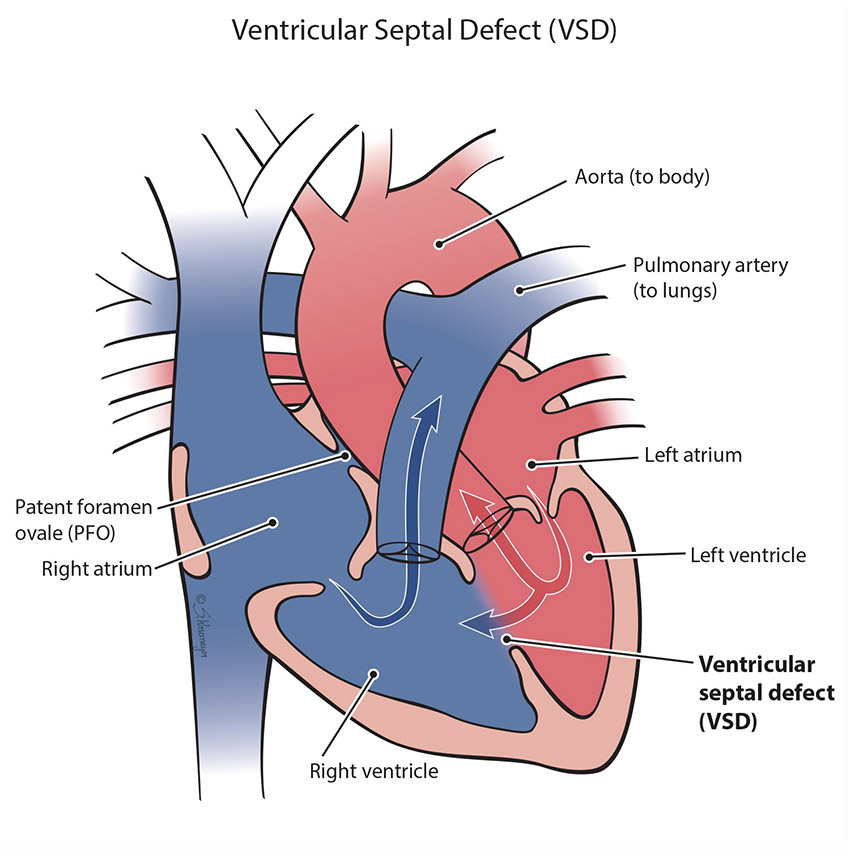
A VSD is a hole in the wall (septum) that separates the right and left bottom pump chambers (ventricles) of the heart. Blood can flow across the hole as the heart pumps. Most times, a baby born with a VSD has no symptoms. However, if the VSD is a large hole, the blood flowing across the hole causes extra blood flow to go to the lungs. This can cause lung problems, trouble breathing, and problems with eating and growth over time.
Treatment for VSD
Most VSDs are small in size and will close on their own as the baby and the heart grow. Large holes need to be closed (repaired) to prevent lifelong complications, particularly growth problems and lung and heart damage. Surgery is usually done by 1 year of age. If a VSD does need to be closed, most patients will need open heart surgery. In surgery, the surgeon will close the VSD with stitches or a patch. Some holes can be closed during a cardiac catheterization (“heart cath”). During this procedure the cardiologist guides a catheter through the blood vessels and goes up to the heart. Once the catheter is in the heart, the VSD is closed with a device called a “septal occluder”.
