Profile of a Cancer: Getting to Know Ewing Sarcoma
Profile of a Cancer: Getting to Know Ewing Sarcoma https://pediatricsnationwide.org/wp-content/themes/corpus/images/empty/thumbnail.jpg 150 150 Katie Brind'Amour, PhD, MS, CHES Katie Brind'Amour, PhD, MS, CHES https://pediatricsnationwide.org/wp-content/uploads/2021/03/Katie-B-portrait.gif- April 19, 2019
- Katie Brind'Amour, PhD, MS, CHES
Ewing sarcoma – a tumor type affecting the bone or soft tissue that primarily affects children and adolescents – has a 5-year survival rate of 70 percent among those with localized disease at diagnosis. Among children whose disease is metastatic, only 30 percent survive 5 years or longer. As a comparison, of all children diagnosed with cancer in the United States, 80 percent survive at least 5 years and many are cured.
What makes Ewing sarcoma so different from other types of common and highly treatable pediatric cancers, such as leukemia, is its primary driver: a chromosomal translocation, called EWS/FLI, that is at the root of about 85 percent of Ewing cases. Although a translocation is not unique to Ewing, it is coupled with a series of other molecular circumstances that together create a challenge in terms of understanding what is going on and how best to treat this cancer type.
Some of the traits that make this sarcoma a current medical challenge also give scientists hope that the disease will, in time, give them great potential to treat not just patients with Ewing, but also those with other cancers.
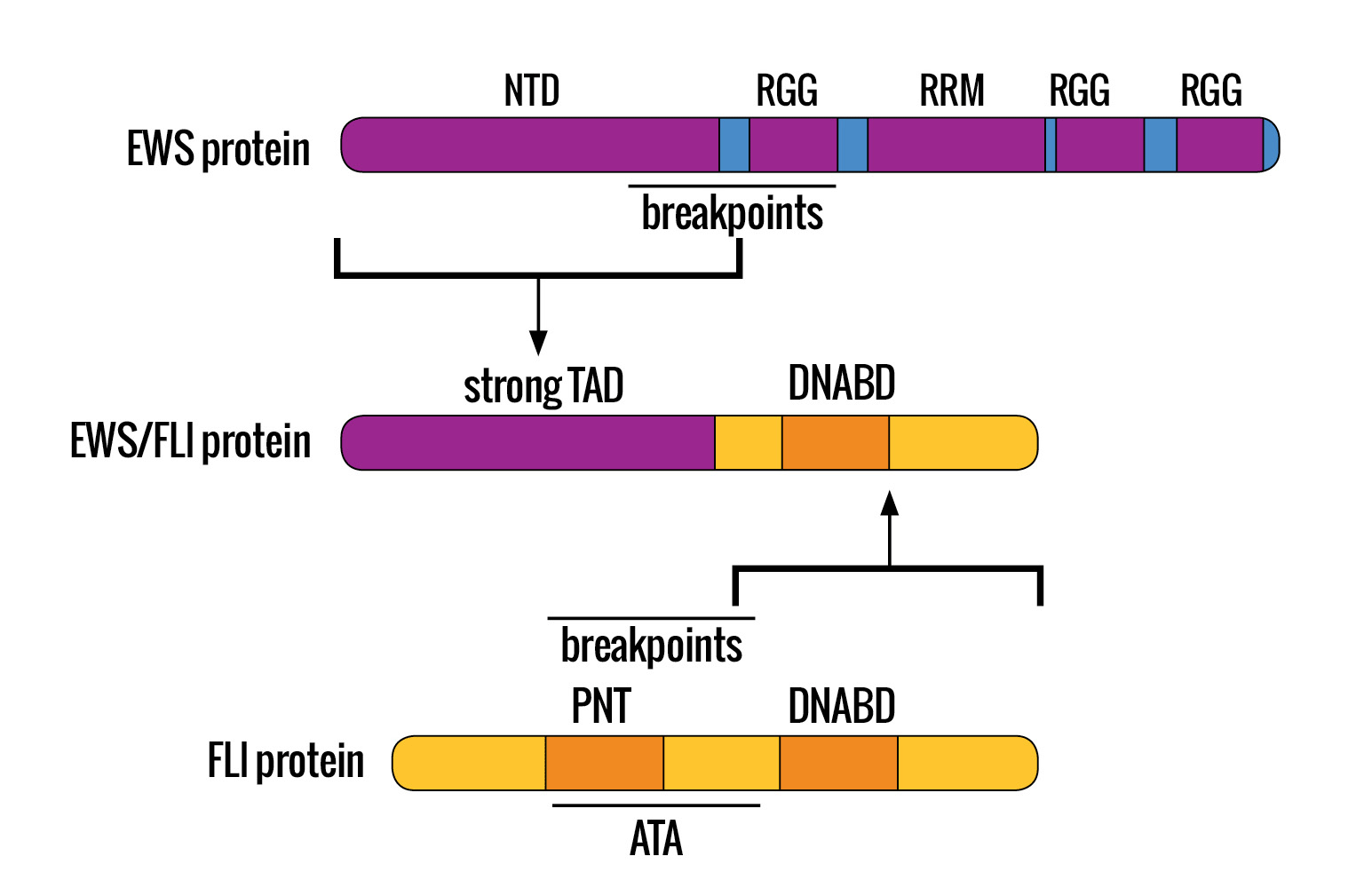
HOW THE CLASSIC EWING SARCOMA TRANSLOCATION OCCURS: The classic oncogenic fusion protein, associated with the vast majority of Ewing sarcomas, arises from a balanced, reciprocal translocation at the molecular genetic level. A portion of the EWS protein fuses to a portion of the FLI protein at the t(11;22)(q24;q12) breakpoint.
WHAT IS EWING SARCOMA?
The father of the field of oncology, James Ewing, MD – the man behind the American Cancer Society and the Journal for Cancer Research – first described his eponymous cancer cells in a published case series in 1921.
The modern characterization of Ewing sarcoma still agrees with Dr. Ewing’s initial description: It affects both flat and long bones, most commonly appearing in the legs and arms, pelvis, trunk, or head and neck. It primarily affects adolescents and requires radiation therapy. Current treatment modalities add in aggressive surgery, chemotherapy and combination therapies, especially in the case of metastatic or recurrent disease.
Ewing sarcoma affects about 10 out of every 1 million people aged 10-19 years. When combined with similar cancer subtypes, Ewing and related sarcomas become the fourth most common cancer among children and young adults.
Unfortunately, Ewing sarcoma is notoriously difficult to treat. Prognosis depends on age at diagnosis, the location of the tumor, metastasis, sex, certain genetic differences in Ewing sarcoma subtypes, and the patient’s cancer-related history, among other traits. Despite many advances in therapy and survival in the past several decades, researchers and clinicians alike have become frustrated with the uphill battle facing Ewing patients.
“We can palliate or comfort patients with metastatic Ewing sarcoma, and hold off the inevitable for a few years, but that’s not enough. Our goal is a cure,” says Timothy Cripe, MD, PhD, chief of the Division of Hematology/Oncology/Blood and Marrow Transplant at Nationwide Children’s Hospital and a clinician-scientist studying Ewing and other pediatric cancers. “It’s incredibly frustrating as a doctor to see patients week after week and know that they’re in trouble.”
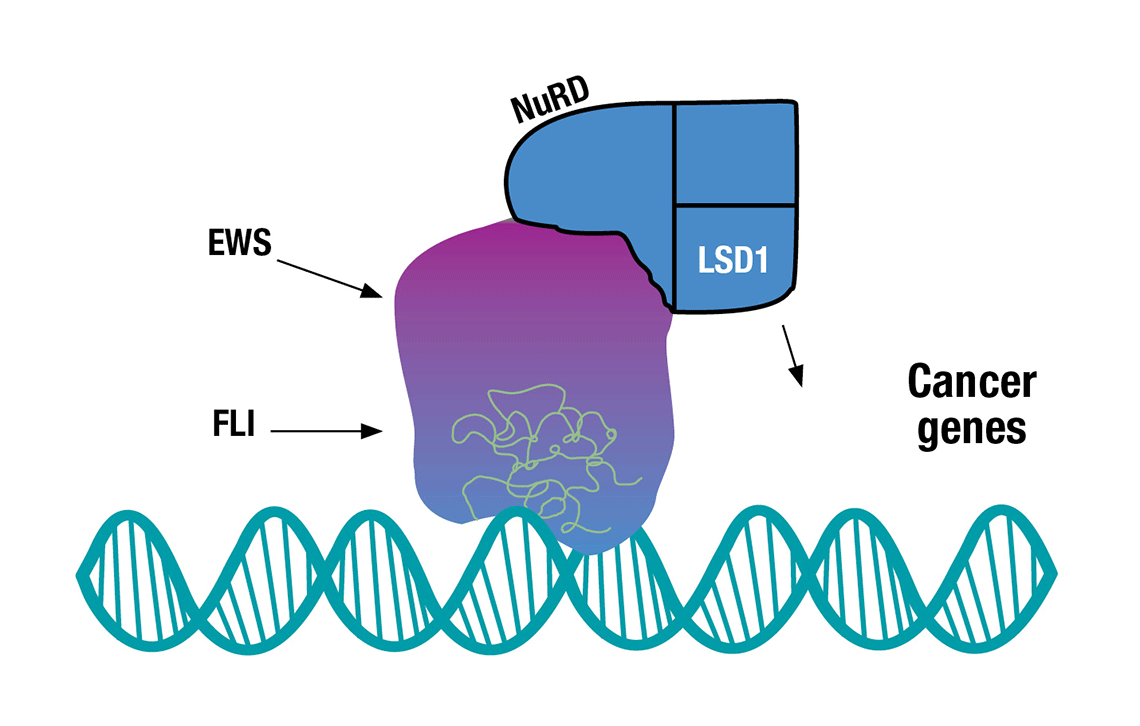
HOW THE FUSION PROTEIN PROMOTES CANCER: The FLI portion of the EWS/FLI fusion protein binds to DNA. When paired with key enzymes (the NuRD and LSD1 complex), the protein appears to physically scrunch up DNA allowing the protein to activate or turn off large batches of genes at a time. The new transcribed and suppressed genes are believed to result in the development of Ewing sarcoma cancer cells.
WHO IS WORKING THE CASE?
Dr. Cripe and his research team have been studying ideal combination therapies for Ewing tumors. Recent publications reveal a promising synergy between two FDA-approved therapies: an oncolytic herpes virotherapy and trabectedin, a chemotherapy drug with a less toxic side effect profile than many currently in use. When given together to a mouse model of Ewing sarcoma, trabectedin appeared to clear out about half of the tumor’s macrophages, which fight infection and spur wound healing. Once macrophage levels dropped, the virotherapy effectively raised inflammation in the tumor, helping the immune system better fight the tumor.
Manipulation of the tumor microenvironment to attack cancer cells is also under study by other investigators. Elizabeth Lawlor, MD, PhD, associate director of education and training at the Rogel Cancer Center and an associate professor in the departments of Pediatrics and Pathology at the University of Michigan, is one of the researchers behind recent studies indicating that the origin of Ewing sarcoma appears to be hijacked stem cells that were originally destined to become bones and supporting soft tissues. Her lab is also analyzing the factors in the tumor’s surrounding cells that trigger the switch from progressive growth as a solitary tumor to migration and metastasis.
“My lab is trying to figure out how cells switch back and forth between different states, and which pathways drive these switches, since we are finding that the ability of Ewing cells to change states is essential for tumor progression,” says Dr. Lawlor. “We want to know how cells come to play different roles at different times. Then if we can find a way to interfere with those transitions, that could be a good way to inhibit tumor survival and prevent metastasis.”
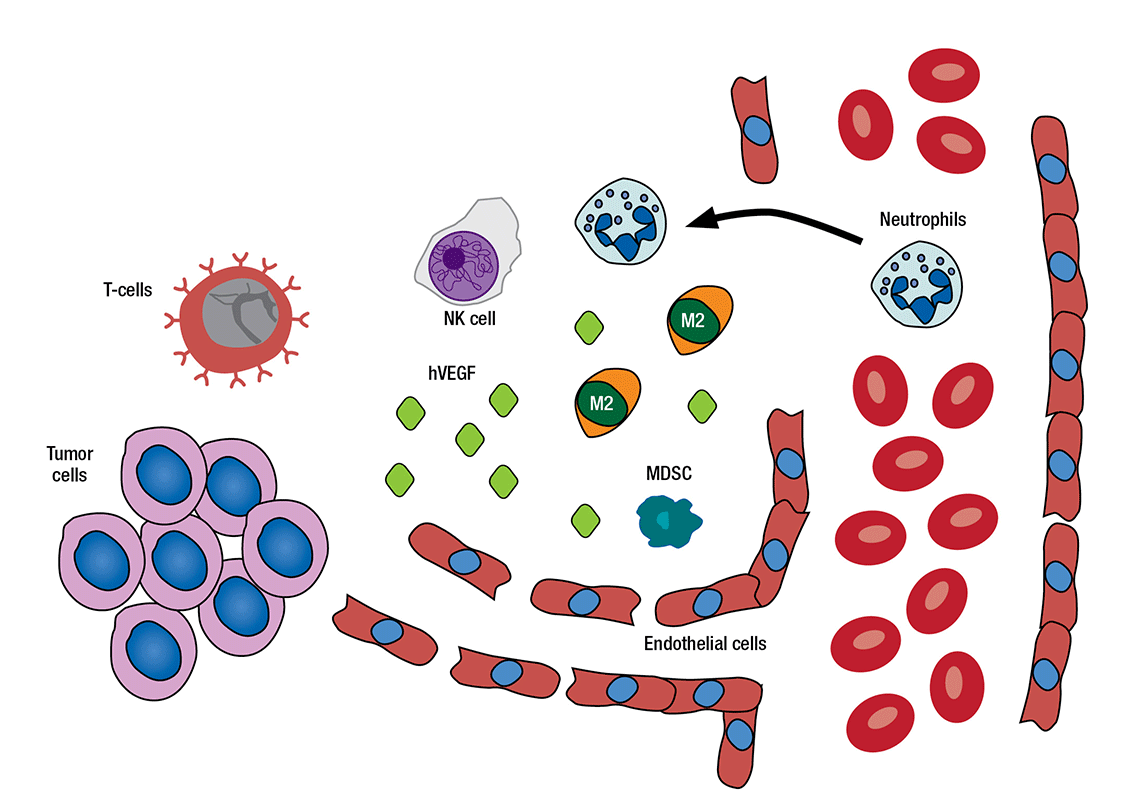
HOW THE MICROENVIRONMENT IMPACTS CANCER CELLS AND VICE VERSA: Some cells found near a tumor, such as T-cells and natural killer cells, fight cancerous cells. Other cells and molecules – such as growth factors, ligands, myeloid-derived suppressor cells (MDSCs) and macrophages – may be recruited or transformed by tumor cells to protect and grow the tumor.
The concept of interference is paramount in numerous research and therapeutic approaches for Ewing sarcoma, and not just in the microenvironment. Other researchers focus on targeting the cancer’s key oncogene and associated subcellular processes: epigenetics, protein binding, coregulatory complexes and DNA transcription. For these approaches, the idea is to hit the cancer as close as possible to the origination of the cancer’s developmental activity as possible, to stop the disease in its tracks, kill existing cancer cells and prevent the creation of new ones. In order to do so, however, scientists must first figure out what is going on inside Ewing sarcoma cells.
For decades, researchers have known the cancer is driven by the mutant EWS/FLI fusion protein. Recent research suggests that the EWS and FLI portions of the fusion protein each have their own characteristics and functions. The FLI portion binds to DNA microsatellites (strings of repetitive DNA previously believed to be “junk” DNA), and the EWS portion binds to groups of proteins and enzymes (called coregulatory complexes) that help the fusion protein regulate transcription.
The microsatellites and EWS/FLI literally seem to be sticky – they start grabbing onto other EWS/FLI oncogenes and microsatellites, pulling portions of DNA together in the cell’s nucleus.
“This is a totally new concept in biochemistry,” says Stephen Lessnick, MD, PhD, director of the Center for Childhood Cancer and Blood Diseases in The Research Institute at Nationwide Children’s. Dr. Lessnick’s lab uncovered the presence and role of microsatellites in Ewing sarcoma and recently received a Cancer Moonshot U54 grant from the National Cancer Institute to continue studying the mechanics of these processes. “Instead of having hundreds of proteins with perfectly complementary surfaces – the exclusive way we have long believed proteins to operate and interact with one another – these sticky microsatellites and EWS/FLI proteins seem to glom onto each other, likely causing the EWS portions to phase separate, similar to oil and water.”
This process likely allows other proteins to interact with EWS/FLI and the microsatellite DNA, which may be important in gene expression. Paired with coregulatory complexes, the EWS/FLI fusion protein and its sticky buddies appear to kick off transcription (by “opening” DNA or making it more accessible) or silence transcription (via the physical squashing of DNA into inaccessible structures) of entire groups of genes at a time, even if they are usually distant from each other.
In his studies on the function of EWS/FLI within the cell and its connections to microsatellites, Dr. Lessnick has also uncovered the critical role of a coregulatory complex called NuRD in enabling EWS/FLI to do its dirty work. When paired with an enzyme called LSD1, NuRD appears to enable EWS/FLI to regulate gene transcription.
“When researchers hear the word ‘enzyme,’ our ears perk up – enzymes have pockets you can drug,” says Dr. Lessnick. When Dr. Lessnick made the discovery of LSD1’s role in Ewing during his time at the University of Utah, he serendipitously had a colleague there – Sunil Sharma, MD, FACP, MBA, deputy director of Huntsman Cancer Institute and the director of the Center for Investigational Therapeutics – who had been studying the use of LSD1 inhibitors in models of breast cancer. Of 100 different types of cell lines Dr. Sharma had tested for sensitivity to the inhibitor drug, Ewing was the most sensitive. Fast-forward a few years, and a clinical trial targeting LSD1 in Ewing with Dr. Sharma’s inhibitor is in progress at multiple children’s hospitals across the country.
Dr. Lessnick hopes that better understanding intracellular mechanics will reveal what type of interference will be deadliest to the activity of the cancer. The physical manipulation of DNA within the nucleus also represents an epigenetic change, which makes Dr. Lessnick eager to explore the possibility of new and existing epigenetic therapies that may help fight the machinery-based cancer processes at work in Ewing sarcoma.
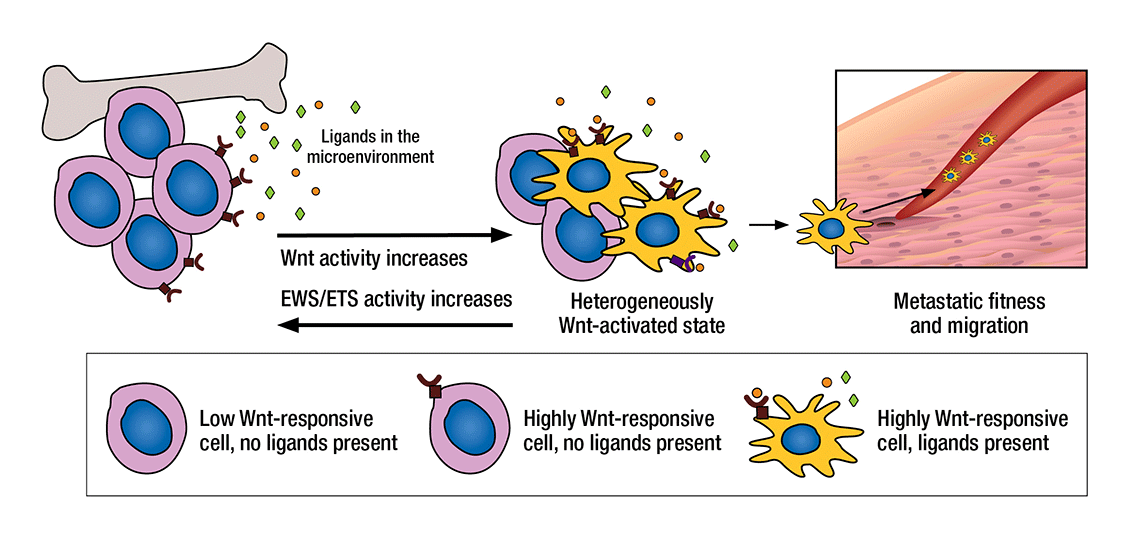
HOW TUMOR CELLS BECOME METASTATIC: Tumor cells may be influenced toward or against metastasis by their microenvironment. When the Wnt/beta-catenin pathway is highly active, for example, receptive tumor cells bind to ligands in the microenvironment that help the cells spur the growth of extracellular matrix, structural collagen, and proteins associated with metastasis. It is believed that the proteins secreted by these activated cells then enhance the cells’ ability to travel throughout the body.
WHERE ARE EWING SARCOMA’S VULNERABILITIES?
To speed along the identification of druggable targets, some teams are focusing on screening Ewing sarcoma for its genomic vulnerabilities. Patrick Grohar, MD, PhD, associate professor and program leader of Skeletal Disease and Cancer Therapeutics in the Van Andel Research Institute’s Center for Cancer and Cell Biology – a chemist by training, prior to his foray into medicine and oncology – cast a wide net in his initial search for potential drug options.
Dr. Grohar set about doing a genome-wide small molecule screen for 50,000 compounds, looking at what kills or turns off the fusion protein, and has screened with other large libraries of compounds as well. His work first revealed that mithramycin inhibits the fusion protein. The screens also demonstrated the EWS-FLI-inhibition abilities of trabectedin – the chemotherapy drug now used by Dr. Cripe in his preclinical studies.
“Ewing sarcoma is driven by a transcription factor. The mantra when I was in med school was that it’s a non-druggable target,” says Dr. Grohar, who is also an oncologist in the Division of Pediatric Hematology/Oncology at Spectrum Health Helen DeVos Children’s Hospital and an associate professor in the Department of Pediatrics at Michigan State University. “But as a chemist I believe everything is druggable. You just have to figure out how to do it.”
After identifying compounds with anti-Ewing potency, Dr. Grohar does in-depth molecular and preclinical testing to determine which may be good drug candidates. Mithramycin is currently in clinical trials, and Dr. Grohar hopes that continued research will reveal more effective second- and third-generation iterations of screen-identified compounds that he can also quickly transition to the clinic.
Taking a similar approach, Kimberly Stegmaier, MD, vice chair of pediatric oncology research and co-director of the Pediatric Hematologic Malignancy Program at the Dana-Farber Cancer Institute, used genome-scale CRISPR-Cas9 screening (a gene editing tool) to find druggable target pathways in TP53 wild-type Ewing sarcoma – a form that differs from many cell line models of the disease but that represents the cancer more accurately as it occurs in most human patients.
Her search revealed multiple possible targets (MDM2, MDM4, USP7 and PPM1D) critically involved in cancer survival, some of which already had compounds available to test for in vitro impact on human Ewing cell lines and mouse models. After successful and synergistic lab-based studies, this p53 pathway activation approach has entered clinical trials in children with Ewing sarcoma and other childhood cancers with a normal TP53 gene. Dr. Stegmaier also has NCI Cancer Moonshot U54 funding to find new therapies for Ewing sarcoma.
To make an even broader view of Ewing sarcoma’s genomic make-up possible, Dr. Lessnick collaborates with teams led by Richard Wilson, PhD, and Elaine Mardis, PhD, co-executive directors of the Institute for Genomic Medicine at Nationwide Children’s, to do a long-read genomic sequence of Ewing sarcoma. One cell line is complete, and Dr. Lessnick has several others in progress or planned.
Ideally, long-read sequencing will reveal the true locations of certain genes and hint at the purpose and weaknesses of various chunks of the cancer’s DNA.
WHY PUT SO MANY RESOURCES INTO EWING SARCOMA?
“Ewing sarcoma serves as a paradigm for a whole series of tumors seen in children, adolescents and young adults,” says Dr. Lessnick. “Better understanding Ewing sarcoma could have direct impact on our ability to treat rhabdomyosarcoma, clear cell sarcoma, desmoplastic small round cell cancers, and a variety of tumors with translocations that generate transcription factors.”
Dr. Stegmaier agrees that discoveries in Ewing sarcoma could mean big advances in other diseases.
“The approaches and technologies that are used to study EWS/FLI could be applied to studying the host of other fusion proteins present in pediatric and adult cancers, such as the TMPRSS2/ERG fusion in the majority of adult prostate cancer tumors,” says Dr. Stegmaier, who is also an attending oncologist at Boston Children’s Hospital.
Recent dramatic successes in chemically targeting kinase fusions, which relate to but are more readily druggable than transcription factors, provide incentive for persevering toward targeting fusions such as EWS/FLI. Moreover, new advances in chemistry offer hope for success in targeting EWS/FLI directly – hence Dr. Stegmaier’s U54 proposal to collaborate heavily with academic chemists in her upcoming research. Once these molecular components are targetable, cancers with similar structural proteins, fusions and transcription factors may also benefit.
The disease also offers researchers a uniquely distraction-free “background” for studying cancer.
“Because Ewing sarcoma in particular, and pediatric cancer in general, are genetically very quiet tumors, not full of thousands of mutations, we can do cleaner genetic and biology studies. These studies are much more challenging with cancers that have scrambled genomes, like you find in most adult carcinomas,” says Dr. Lawlor. This fact also gives Dr. Lawlor hope that Ewing may, when the right biology is understood, become highly treatable.
“The concept is that pediatric cancers can be considered developmental disorders, created when plastic stem cells are hijacked to become a sort of cancer stem cell,” says Dr. Lawlor. “You don’t need 50 mutations to create a cancer stem cell, you just need a couple to give a normal stem cell malignant properties. It didn’t take as much to create them, and so hopefully it will not take as much to dismantle them.”
WHEN WILL NEW THERAPIES REACH PATIENTS?
Drs. Lessnick, Stegmaier, and Grohar have Ewing-related clinical studies underway, and Dr. Cripe’s and Dr. Lawlor’s research is expected to hit the clinic in the next few years.
Experts in Ewing sarcoma research agree that treatment advances will, at least in the near future, involve sophisticated regimens of therapy administration and carefully timed and dosed combinations of epigenetic and chemotherapy drugs. They are also working to combat the toxicity of current therapies, as many surviving Ewing patients suffer from long-term treatment-related side effects.
“We have a great field in Ewing sarcoma because we all are willing to talk to each other – we share our unpublished data and what we’re thinking,” says Dr. Lessnick. “Our collaborative, open approach will make a big difference in how quickly we make a difference for patients.”
This story was published in the Spring/Summer 2019 print issue. Download the full issue.
References:
- Cripe TP. Ewing sarcoma: An eponym window to history. Sarcoma. 2011;2011:457532.
- Currier MA, Eshun FK, Sholl A, Chernoguz A, Crawford K, Divanovic S, Boon L, Goins WF, Frischer JS, Collins MH, Leddon JL, Baird WH, Haseley A, Streby KA, Wang PY, Hendrickson BW, Brekken RA, Kaur B, Hildeman D, Cripe TP. VEGF blockade enables oncolytic cancer virotherapy in part by modulating intratumoral myeloid cells. Molecular Therapy. 2013 May;21(5):1014-23.
- Denton NL, Chen CY, Hutzen B, Currier MA, Scott T, Nartker B, Leddon JL, Wang PY, Srinivas R, Cassady KA, Goins WF, Cripe TP. Myelolytic treatments enhance oncolytic herpes virotherapy in models of Ewing sarcoma by modulating the immune microenvironment. Molecular Therapy Oncolytics. 2018 Oct 18;11:62-74.
- Pishas KI, Drenberg CD, Taslim C, Theisen ER, Johnson KM, Saund RS, Pop IL, Crompton BD, Lawlor ER, Tirode F, Mora J, Delattre O, Beckerle MC, Callen DF, Sharma S, Lessnick SL. Therapeutic targeting of KDM1A/LSD1 in Ewing sarcoma with SP-2509 engages the endoplasmic reticulum stress response. Molecular Cancer Therapy. 2018 Sep;17(9):1902-1916.
- Johnson KM, Mahler NR, Saund RS, Theisen ER, Taslim C, Callender NW, Crow JC, Miller KR, Lessnick SL. Role for the EWS domain of EWS/FLI in binding GGAA-microsatellites required for Ewing sarcoma anchorage independent growth. Proceedings of the National Academies of Science USA. 2017 Sep 12;114(37):9870-9875.
- Krook MA, Hawkins AG, Patel RM, Lucas DR, Van Noord R, Chugh R, Lawlor ER. A bivalent promoter contributes to stress-induced plasticity of CXCR4 in Ewing sarcoma. Oncotarget. 2016 Sep 20;7(38):61775-61788.
- Pedersen EA, Menon R, Bailey KM, Thomas DG, Van Noord RA, Tran J, Wang H, Qu PP, Hoering A, Fearon ER, Chugh R, Lawlor ER. Activation of Wnt/beta-Catenin in Ewing sarcoma cells antagonizes EWS/ETS function and promotes phenotypic transition to more metastatic cell states. Cancer Research. 2016 Sep 1;76(17):5040-53.
- Hawkins AG, Basrur V, da Veiga Leprevost F, Pedersen E, Sperring C, Nesvizhskii AI, Lawlor ER. The Ewing sarcoma secretome and its response to activation of Wnt/beta-catenin signaling. Molecular and Cellular Proteomics. 2018 May;17(5):901-912.
- Grohar PJ, Glod J, Peer CJ, Sissung TM, Arnaldez FI, Long L, Figg WD, Whitcomb P, Helman LJ, Widemann BC. A phase I/II trial and pharmacokinetic study of mithramycin in children and adults with refractory Ewing sarcoma and EWS-FLI1 fusion transcript. Cancer Chemother Pharmacol. 2017 Sep;80(3):645-652.
- Stolte B, Iniguez AB, Dharia NV, Robichaud AL, Conway AS, Morgan AM, Alexe G, Schauer NJ, Liu X, Bird GH, Tsherniak A, Vazquez F, Buhrlage SJ, Walensky LD, Stegmaier K. Genome-scale CRISPR-Cas9 screen identifies druggable dependencies in TP53 wild-type Ewing sarcoma. Journal of Experimental Medicine. 2018 Aug 6;215(8):2137-2155.
- Iniguez AB, Stolte B, Wang EJ, Conway AS, Alexe G, Dharia NV, Kwiatkowski N, Zhang T, Abraham BJ, Mora J, Kalev P, Leggett A, Chowdhury D, Benes CH, Young RA, Gray NS, Stegmaier K. EWS/FLI confers tumor cell synthetic lethality to CDK12 inhibition in Ewing sarcoma. Cancer Cell. 2018 Feb 12;33(2):202-216.e6.
Image credits: Nationwide Children’s
About the author
Katherine (Katie) Brind’Amour is a freelance medical and health science writer based in Pennsylvania. She has written about nearly every therapeutic area for patients, doctors and the general public. Dr. Brind’Amour specializes in health literacy and patient education. She completed her BS and MS degrees in Biology at Arizona State University and her PhD in Health Services Management and Policy at The Ohio State University. She is a Certified Health Education Specialist and is interested in health promotion via health programs and the communication of medical information.
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 28, 2014